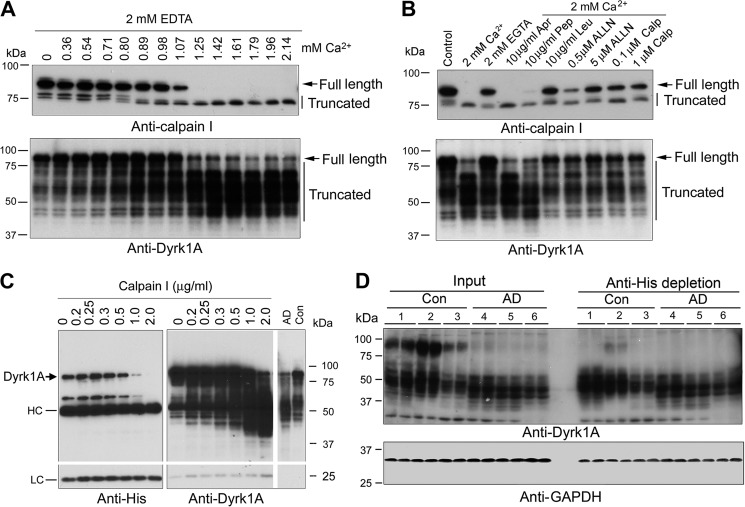
FIGURE 3.
Dyrk1A is proteolyzed by calcium-mediated truncation/activation of calpain I. A, calcium-activated proteolysis of calpain I and Dyrk1A in the human brain extract. Normal human brain frontal cortical extract was incubated at 30 °C for 10 min in the presence of 2.0 mm EDTA and various concentrations (0.00–2.14 mm) of CaCl2. Then the incubated tissue extract was analyzed by Western blots developed with anti-calpain I or anti-Dyrk1A. B, selective inhibition of calcium-activated proteolysis of calpain I and Dyrk1A. Normal human frontal cortical extract was incubated at 30 °C for 10 min in the presence of 2.0 mm each of EDTA and CaCl2 plus various selective protease inhibitors, as indicated in the figure, followed by Western blots probed with anti-calpain I or anti-Dyrk1A (8D9, against aa 91–151) to detect the proteolysis. An arrow indicates the full-length calpain I (top) or Dyrk1A (bottom); vertical lines indicate the truncated calpain I or Dyrk1A. Apr, aprotinin; Pep, pepstatin; Leu, leupeptin; Calp, calpestatin peptide. C, proteolysis of Dyrk1A by calpain I. Dyrk1A was immunoprecipitated by anti-His from HEK-293FT cells that overexpressed Dyrk1A and incubated with various concentration of calpain I in the presence of CaCl2 for 10 min at 30 °C, followed by Western blot analyses. AD, AD brain; Con, control brain; HC, heavy chain of antibody; LC, light chain of antibody. D, depletion of the full-length but not truncated Dyrk1A from human brain extract by anti-His antibody. Human brain frontal cortical extracts from AD and control cases were incubated with anti-His precross-linked protein G-agarose beads overnight at 4 °C. The same volumes of the original brain extracts and of those after immunodepletion were subjected to Western blots developed with anti-Dyrk1A (8D9) and anti-GAPDH. The depletion of full-length Dyrk1A by anti-His from AD brain indicated cleavage of Dyrk1A upstream of the histidine domain.