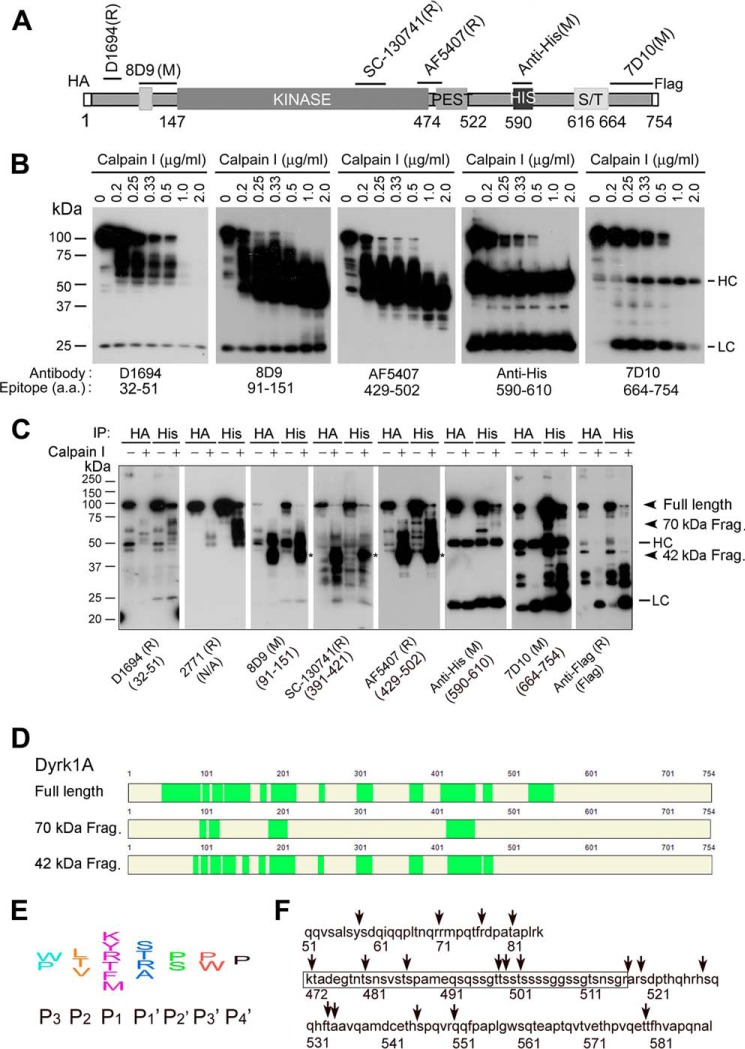
FIGURE 4.
Proteolysis of Dyrk1A by calpain I at the C terminus precedes that at the N terminus. A, diagram of recombinant Dyrk1A and epitopes of the Dyrk1A antibodies used in this study. B, Western blots of proteolyzed Dyrk1A. Dyrk1A was immunoprecipitated by anti-HA from HEK-293FT cells that overexpressed Dyrk1A and was incubated with various concentrations of calpain I in the presence of CaCl2 for 10 min at 30 °C, followed by Western blots developed with antibodies indicated below the blots. C, cleavage of Dyrk1A by calpain I in vitro at both the C terminus and N terminus. Dyrk1A tagged with HA at the N terminus and with FLAG at the C terminus was immunoprecipitated by anti-HA or anti-His and then incubated with calpain I for 10 min. The proteolyzed products were analyzed by Western blots developed with antibodies indicated below the blots. D, mass spectrometry of truncated Dyrk1A. Truncated Dyrk1A by calpain I was separated by SDS-PAGE, and the full-length, ∼70-kDa, and ∼42-kDa Dyrk1A bands were subjected to MS analysis. The MS-detected peptides are marked by green color. E, diagram of the consensus sequences for calpain cleavage. F, predicted cleavage sites by calpain. Arrows indicate possible cleavage sites of Dyrk1A by calpain I according to its consensus cleavage sites. The framed sequence is the PEST region.