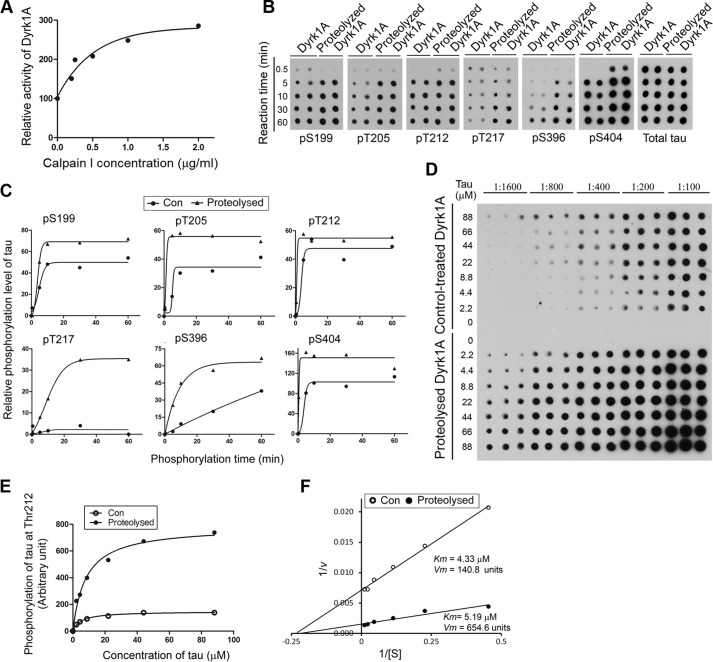
FIGURE 5.
Proteolysis of Dyrk1A by calpain I enhances its kinase activity. A, increase of Dyrk1A activity by proteolysis with calpain I. Immunopurified Dyrk1A with anti-His from Dyrk1A-overexpressing HEK-293FT cells was proteolyzed with various concentrations of calpain I as described in C. After adding ALLN to inhibit proteolysis, the kinase activity of proteolyzed Dyrk1A was assayed toward Tau441 as a substrate in the presence of a calpain inhibitor, ALLN. B and C, increased site-specific phosphorylation of Tau by proteolyzed Dyrk1A. Immunopurified Dyrk1A was proteolyzed with 2.0 μg/ml calpain 1 for 10 min at 30 °C. After adding 50 μm ALLN to terminate the proteolysis reaction, the proteolyzed Dyrk1A and control-treated Dyrk1A (no calpain I) were incubated with Tau (0.2 mg/ml) for various periods at 30 °C in the reaction buffer to phosphorylate Tau. The phosphorylated Tau products were subjected to immuno-dot blots developed with phosphorylation-dependent and site-specific Tau antibodies and total Tau antibody (B). Tau phosphorylation at each individual site was then quantified densitometrically, and the relative levels after being normalized with the total Tau levels were plotted against reaction times. Each curve was fitted with GraphPad Prism version 5 by a Boltzmann sigmoidal program (C). D, enzyme kinetics of Dyrk1A-catalyzed Tau phosphorylation. Various concentrations of Tau441 were incubated with either the proteolyzed Dyrk1A or the control-treated Dyrk1A (no calpain I) at 30 °C for 10 min, and Tau phosphorylation at Thr212 in the reaction mixtures was determined by immuno-dot blots. E and F, the blots shown in C were quantified densitometrically, and the data were plotted against Tau concentration and fitted with GraphPad Prism version 5 by a Michaelis-Menten program (E) or as a Lineweaver-Burk plot (F), and Km and Vmax were determined from the latter.