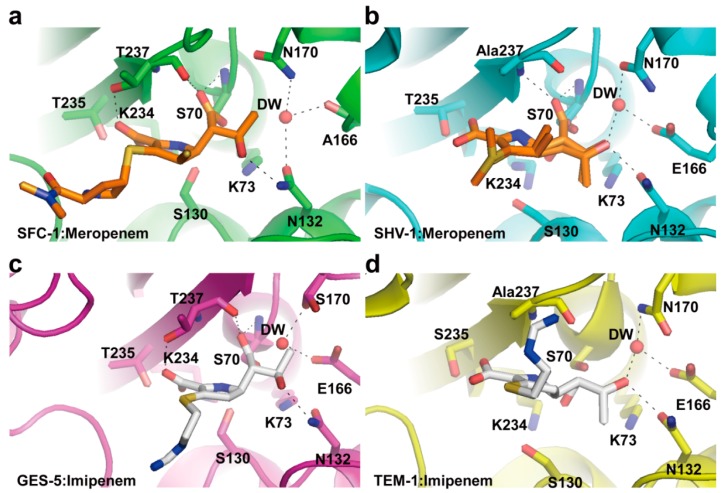
Figure 4.
Comparison between carbapenem acyl-enzymes of class A carbapenemases (SFC-1 and GES-5) and non-carbapenemases (SHV-1 and TEM-1). (a) SFC-1 (E166A mutant):meropenem (PDB entry 4EV4, green); (b) SHV-1:meropenem (PDB entry 2ZD8, cyan); (c) GES-5:imipenem (PDB entry 4H8R, magenta); and (d) TEM-1:imipenem (PDB entry 1BT525, yellow). The residues (S70, K73, S130, N132, E166/A166, N170/S170, T235, and T237) in the active-site cleft are shown as sticks. Meropenem and imipenem carbon atoms are rendered in orange and white, respectively. Hydrogen bonds involving the deacylating water molecule (DW, red sphere), the acyl-enzyme carbonyl group, the carbapenem C3 carboxylate, the 6α-1R-hydroxyethyl group of carbapenem, and Asn132 NH2 are indicated by dashed black lines. These figures were prepared using PyMOL [54] and data adapted from Fonseca et al. [37].