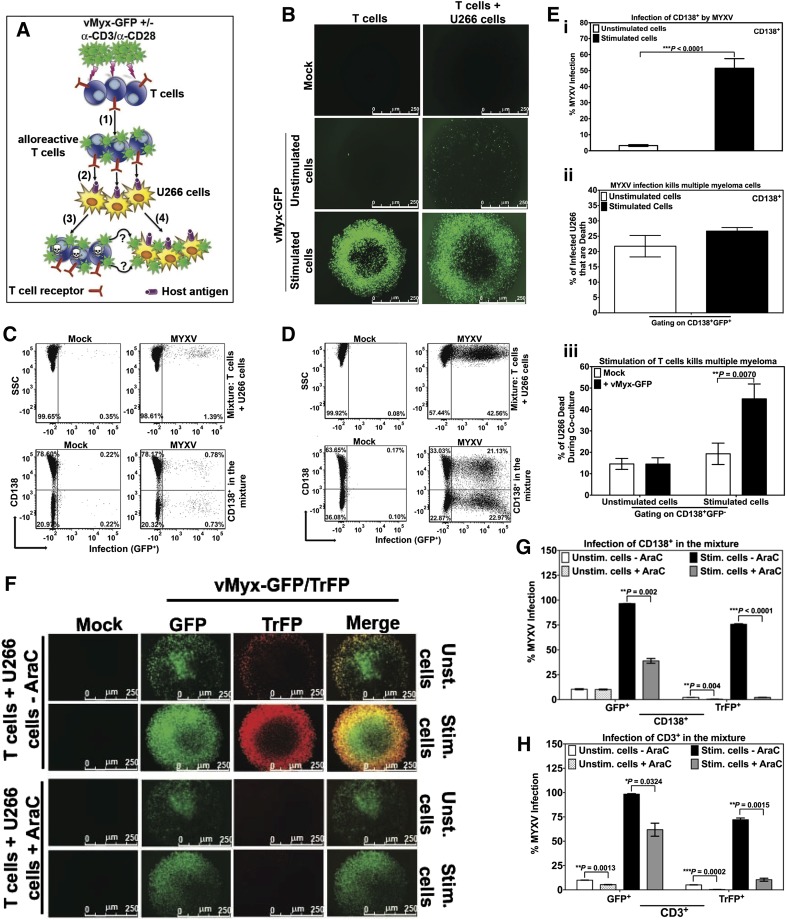
Figure 7.
Input MYXV and virus progeny from activated human T cells are both efficiently transferred to human multiple myeloma cells. To investigate whether MYXV infection of unstimulated vs activated T cells can secondarily target and infect virus-susceptible human U266 MM cells, an in vitro virus transfer assay was performed and is described in diagram (A). Experimental schematic depicting human T lymphocytes incubated with MYXV in the presence or absence of activating anti-CD3/CD28 microbeads, (1) MYXV binding to T cells (alloreactive T cells), free MYXV washed from culture, (2) admixture of human MM (U266 cells). As a result a dual action of MYXV is proposed: (3) MYXV mediates infection/suppression of alloreactive T cells when they interact with host U266 antigens, and (4) the infection of malignant cells by passing of virus from activated T cells to myeloma cells (GFP+). (B) Fluorescence micrographs showing minimal increase in MYXV infection (GFP+) in unstimulated conditions when T cells were mixed with MM cells (middle panels). However, after stimulation there was a significant increase in MYXV infection of all cells (bottom panels). (C) Flow cytometry was used to quantify infection in cell subsets. CD138+ myeloma cells showed only minimal MYXV infection in unstimulated conditions (bottom right plot). (D) In stimulated conditions, there was a significant increase in the percentage of CD138+ myeloma cells with MYXV infection (bottom right panel). Compared with unstimulated conditions, activated T lymphocytes caused more than a 25-fold increase in myeloma infection with MYXV (from 0.78% to 21.13%). (E) (i) Bar graph showing percentage of the CD138+ myeloma cell population with MYXV infection in the unstimulated (white) vs stimulated (black) conditions. (ii) Bar graph showing the percentage of MM dead (CD138+) induced by MYXV infection (GFP+) (ie, gating on CD138+GFP+) under unstimulated (white) vs stimulated (black) conditions (ie, 21% vs 27%, respectively). (iii) Gating on CD138+GFP−, bar graph showing percentage of MM dead (CD138+). Mock-treated (white), or MYXV-treated (black) T cells and under stimulation with anti-CD3/CD28 resulted on 19.30% and 44.96% of MM dead, respectively. On the other hand, mock-treated (white), MYXV-treated (black) T cells without anti-CD3/CD28, resulted in <15% of MM dead. T cells from 3 different donors were tested and showed reproducibly consistent virus-transfer and killing results. (F) To determine whether progeny and/or input virus is transferred from activated T cells to MM cells, late gene expression, and therefore the generation of progeny MYXV, was blocked by using AraC. Briefly, after exposure of T cells with vMyx-GFP/TrFP, 10 μg/mL AraC (+AraC) or vehicle only (-AraC) were added to the T cells with or without α-CD3/α-CD28 stimulation. After 48 hours of culturing T cells ± AraC, U266 cells were added to the culture and incubated at 37°C 5% CO2 for an additional 48 hours (see supplemental Methods for more details). Infection was evaluated using florescence microscopy. Flow cytometry was used to quantify the levels of infection of (G) MM cells (CD138+) and (H) T cells (CD3+). Data indicate that after infection of activated T cells, MYXV replicates, but both input virus (unaffected by AraC) and virus progeny (inhibited by AraC) are both then transferred to myeloma cells to initiate infection of these cells. At least 3 independent experiments were performed.