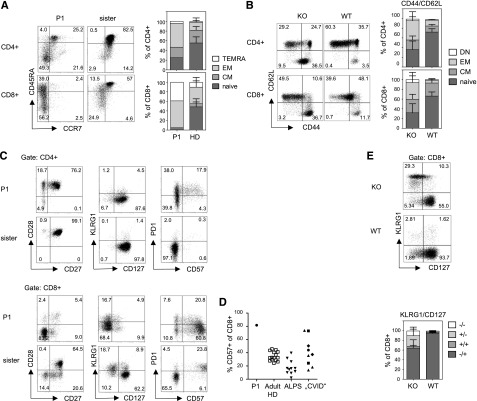
Figure 2.
Advanced differentiation of TPP2-deficient T cells. (A) Left panel: peripheral blood mononuclear cells (PBMC) of the patient (P1, age 11 years) and his asymptomatic heterozygous sister (age 7 years) were analyzed for expression of the differentiation markers CCR7 and CD45RA (gated on CD3+CD4+ and CD3+CD8+ T cells, respectively). Right panel (human PBMC): Proportions of naïve (CCR7+CD45RA+, naive), central memory (CM) (CCR7+CD45RA−), effector memory (EM) (CCR7−CD45RA−), and terminal differentiated T-effector memory cells reexpressing CD45RA (TEMRA) (CCR7−CD45RA+) among CD4+ or CD8+ T cells of the patient (P1) compared with adult healthy donors (HD) (n = 20). (B) Left panel: Splenocytes of wild-type (WT) and TTP2-deficient knockout mice (KO) were analyzed for CD62L and CD44 expression (gated on CD3+CD4+ and CD3+CD8+ T cells). Right panel (mouse splenocytes): Proportions of naïve (CD44−CD62L+), CM (CD44+CD62L+), EM (CD44+CD62L−), and double-negative (DN) (CD44−CD62L−) cells were determined among CD4+ or CD8+ T cells of 6 to 12 months old TPP2 deficient knockout mice (KO) (n = 16) compared with age-matched WT mice (n = 7). (C) Expression of the indicated activation/differentiation markers on CD4 and CD8 T cells of the patient (P1) and his healthy heterozygous sister. (D) Percentage of CD57 expressing CD8 T cells in the patient (P1) compared with adult healthy controls, FAS mutant patients with ALPS (pediatric and adult), and pediatric patients with autoimmune cytopenia and lymphoproliferation in the context of CVID. Of these, CVID patients, 2 had LRBA deficiency (diamonds), 2 had activating PIK3CD mutations (squares), and 1 had a CTLA4 mutation (circle). (E) Expression of KLRG1 and CD127 on CD8 T cells of TPP2-deficient (KO) and age-matched WT mice.