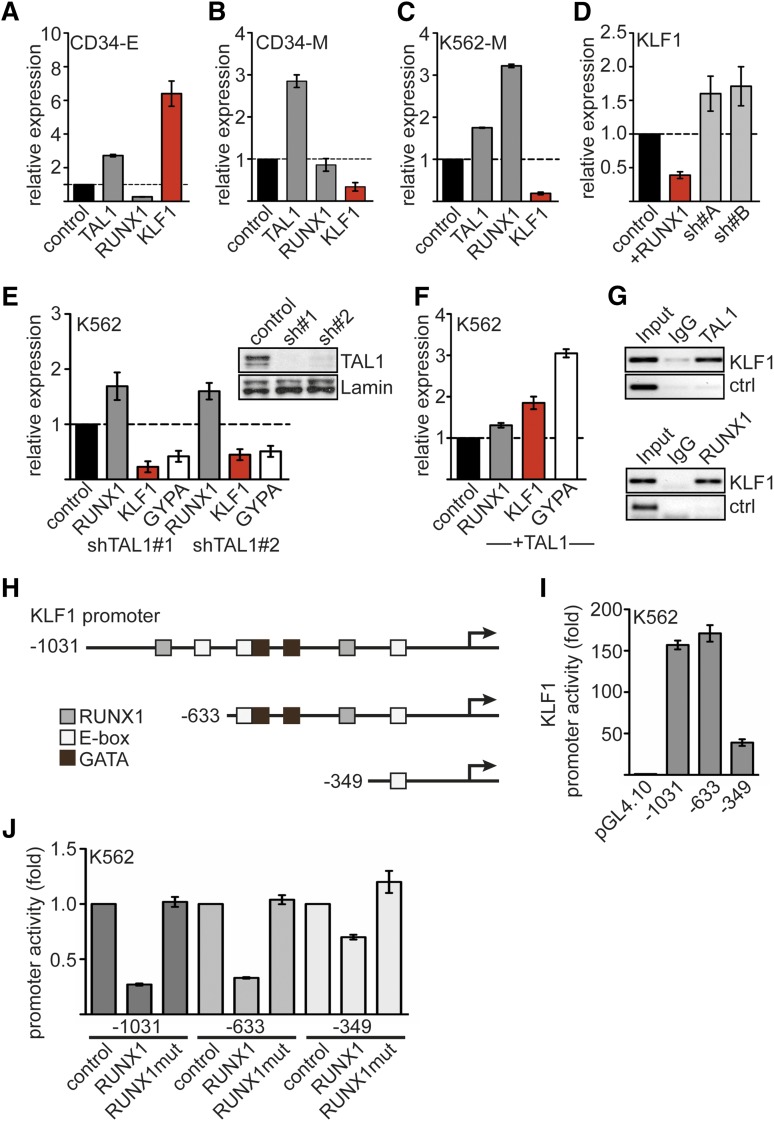
Figure 2.
Expression of RUNX1, TAL1, and KLF1. Expression of TAL1, RUNX1, and KLF1 on erythroid or megakaryocytic differentiation of hCD34+ hematopoietic progenitor cells. Human expanded CD34+ cells were differentiated toward erythrocytes (CD34-E) (A) or megakaryocytes (CD34-M) (B). Shown is the relative mRNA expression compared with control cells. (C) Expression of TAL1, RUNX1, and KLF1 upon megakaryocytic differentiation of K562 cells. (D) Overexpression of RUNX1 in K562 cells reduces KLF1 expression compared to the empty vector control. RUNX1 knockdown using 2 different shRNAs increased KLF1 expression. (E) TAL1 knockdown by shRNA in K562 cells reduces KLF1 and GYPA mRNA expression. TAL1 was knocked down with 2 different shRNAs. The western blot shows the decreased TAL1 protein amount. Lamin was used as a loading control. (F) TAL1 overexpression in K562 cells increases KLF1 and GYPA mRNA expression. (G) KLF1 is a direct target gene of TAL1 and RUNX1. ChIP in K562 cells with antibodies against TAL1 (top) and RUNX1 (bottom) shows binding of the transcription factors to the KLF1 promoter but not to a control region. (H) Schematic representation of the KLF1 promoter region. Predicted binding sites for RUNX1, TAL1 (E-box), and GATA are shown. Deletion constructs for promoter analysis are displayed. The arrows mark the transcriptional start site. (I) The activity of the KLF1 promoter deletion constructs in K562 cells is shown as fold change related to the empty luciferase vector. (J) KLF1 promoter assay reveals inhibitory effect of RUNX1 on the activity of KLF1 promoter luciferase constructs −1031 and −633 but not −349. RUNX1mut did not influence promoter activity. Normalized luciferase values are shown in fold change related to the promoter construct without RUNX1 transfection. Error bars represent the standard deviation from at least 4 determinations. Ig, immunoglobulin.