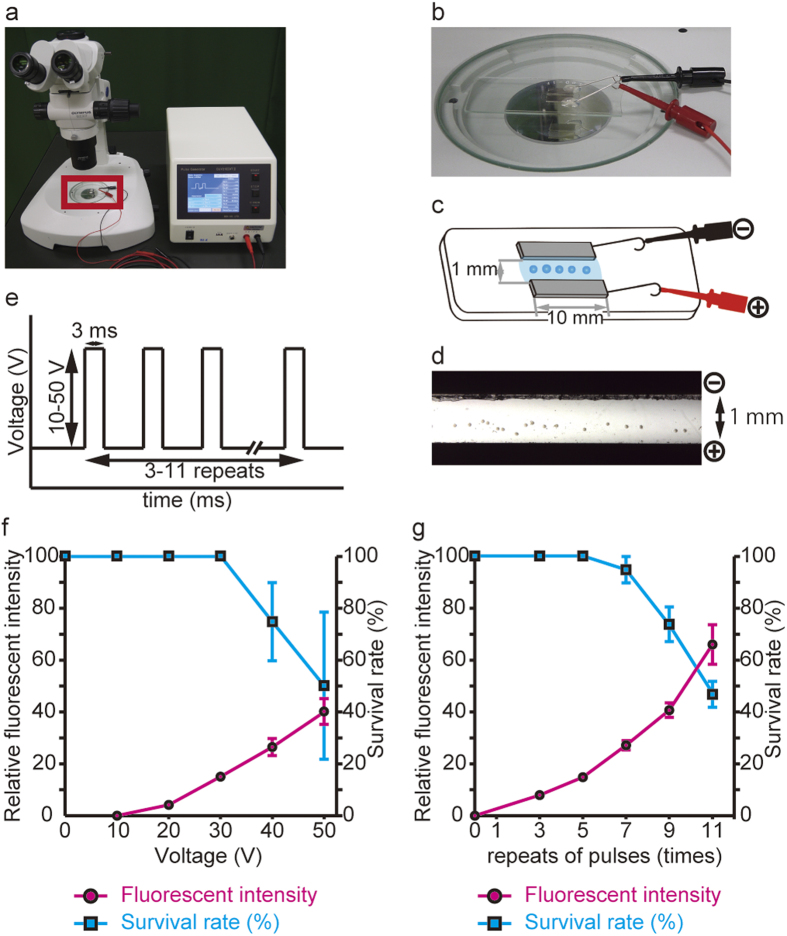
Figure 1. Optimal conditions for the electroporation used to deliver mRNA into fertilized eggs.
(a) Electroporation set-up used in this study. The right box is the electroporator CUY21 EDIT II (BEX Co. Ltd.), which generates electric pulses, and the left is a stereoscopic microscope for embryo manipulation. Two electrodes were placed on the microscope stage and connected to the electroporator. (b) Higher magnification of the red rectangle in (a). (c) Schematic of the electroporation chamber with customized platinum electrodes (BEX Co. Ltd.). Fertilized mouse eggs were placed in the RNA solution in the gap between the electrodes. (d) Microscopic view of the eggs set in the electrode gap. The eggs were manually positioned into a line prior to electroporation. (e) Schematic of the electroporation conditions used to introduce RNAs into mouse eggs: three to eleven repeats of a square pulse of 10–50 V; 3-msec pulses with 97-msec intervals were used. (f) Fluorescence intensity of mCherry (red circles) and rate of electroporated embryo survival to the blastocyst stage (blue squares) were plotted at various voltages (n = 10). The duration of each pulse and number of pulse repeats were fixed at 3 msec and 5 repeats, respectively. (g) The fluorescent intensity of mCherry (red closed circle) and the survival rate of the electroporated embryos to the blastocyst stage (blue squares) were plotted as a function of the number of electroporation repeats (n = 12). The voltage and duration of each pulse were fixed at 30 V and 3 msec, respectively.