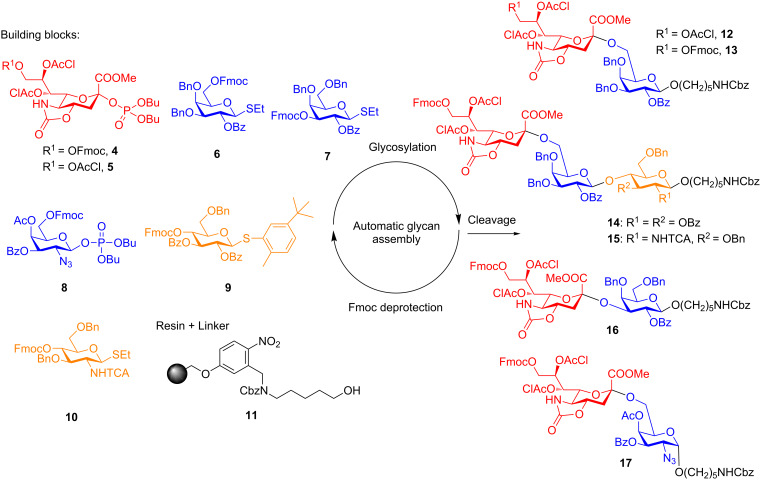
Scheme 2.
Automated synthesis of oligosaccharides with α(2,3)-, α(2,6)-sialic acid linkages. Glycosylations: a) 2 × 5 equiv TMSOTf, ACN/DCM (1:1), −50 °C (5 min), −30 °C (10 min), −20 °C (80 min), −10 °C (10 min), 0 °C (10 min) for 4 and 5. b) 2 × 5 equiv TfOH, NIS, DCM, −40 °C (5 min), −20 °C (30 min) for 6 and 7. c) 2 × 5 equiv TMSOTf, DCM/dioxane (3:2), 20 °C (90 min), for 8. d) 2 × 5 equiv TfOH, NIS, DCM, −30 °C (5 min), −10 °C (25 min) for 9 and 10. Fmoc Deprotection: e) 3 × 20% NEt3 in DMF, 5 min. Photocleavage: f) UV irradiation using a continuous flow reactor, DCM, rt. Synthesis of 12 or 13: (1) 6, b, (2) e, (3) 4 or 5, a (4) f, 30% for four steps to yield 12, 40% for four steps to yield 13; synthesis of 14: (1) 9, d, (2) e, (3) 6, b, (4) e, (5) 4, a, (6) f, 22% for six steps; synthesis of 15: (1) 10, d, (2) e, (3) 6, b, (4) e, (5) 4, a, (6) f, 7% for six steps; synthesis of 16: (1) 7, b, (2) e, (3) 4, a, (4) f, 19% for four steps; synthesis of 17: (1) 8, c, (2) e, (3) 3 × Ac2O, py, 25 °C for 60 min, (4) 4, a, (5) f, 10% for five steps.