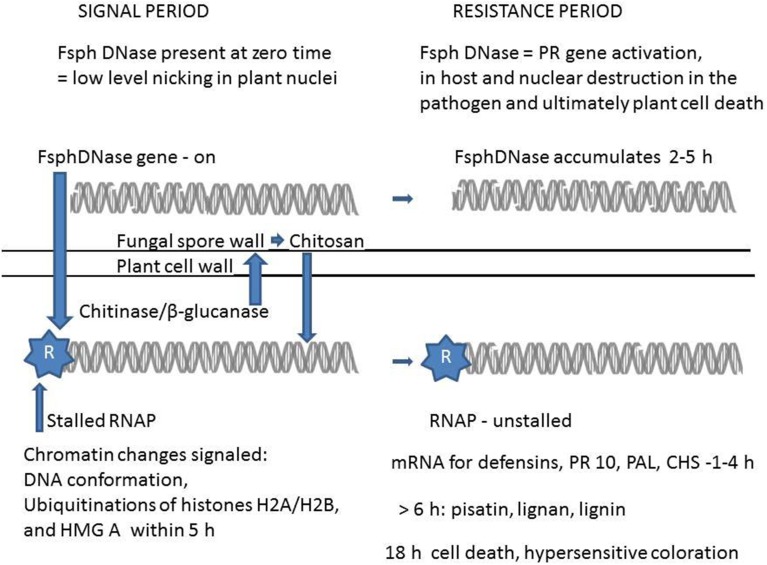
Figure 1.
A proposed scheme relating the role of DNase in altering plant chromatin and subsequently enhancing transcription and cell death. The upper portion of the scheme relates the alterations of DNA within the fungal cell. At zero time the DNA helix is nearly intact in the germinating Fusarium solani f. sp. phaseoli (Fsph) spore, however FsphDNase is present and released as the spore germination begins. Following the entrance of FsphDNase into the plant cell (lower portion) there is a low level nicking of the DNA within the plant nucleus. The subsequent loosening of the DNA helical structure within the nucleosome assembly along with the ubiquitination of nuclear proteins, histone H2A/H2B and the architectural transcription factor (HMG A) frees the stalled RNA polymerase II complex (RNAP) enabling the defense genes to be transcribed. Of the pathogenesis-related (PR type) genes expressed are the defensins, (DRR 39, DRR230) with direct potent antifungal activity. PR10 (DRR49) codes a putative RNase protein. Increases in phenylalanine ammonia lyase (PAL) and chalcone synthetase (CHS) are secondary plant enzymes that are essential for fungal suppressive components such as pisatin, lignan, and lignin. Since resistance to Fsph the inappropriate pathogen is complete in 6 h pi, the after 6 h appearance of these latter components and expression of cell death and hypersensitivity are not likely major contributors to disease resistance. The plant constitutively produces background levels of chitinase and β-glucanase. Induced levels appear starting 10 h pi. These secretable enzymes attack the regions of fungal wall chitin that is 80% N-acetyl-glucosamine and fungal wall β-glucan. Heptamers of chitosan oligomers are released that both induce PR genes and inhibit fungal growth. Within 2 h of contact with the plant tissue the nuclei within the growing tip of the germinating Fsph spore undergo DNA degradation. This disruption is adequate to prevent further fungal growth.