Abstract
Biodegradable and biocompatible polymers are widely used for the encapsulation of drug molecules. Various particulate carriers with different sizes and characteristics have been prepared by miscellaneous techniques. In this review, we reported the commonly used preformed polymer based techniques for the preparation of micro and nano-structured materials intended for drug encapsulation. A description of polymer-solvent interaction was provided. The most widely used polymers were reported and described and their related research studies were mentioned. Moreover, principles of each technique and its crucial operating conditions were described and discussed. Recent applications of all the reported techniques in drug delivery were also reviewed.
Keywords: drug delivery, particles, polymer, encapsulation, carriers, operating conditions
Introduction
Particulate carriers have gained tremendous interest during the last decades which permitted to deliver many hydrophilic and hydrophobic molecules. Obtained particles present small size which facilitates their absorption. These drug delivery systems protect active pharmaceutical ingredients from degradation, enhance biopharmaceutical properties and could provide passive or active targeting or sustained delivery. Biomedical applications of the developed carriers are continuously growing (Ahmad, 2013[3]; Soares, 2013[229]; Miladi et al., 2013[177]). Although, they present different physicochemical properties, the used polymers are mainly biocompatible and biodegradable. A multitude of techniques are used to obtain these particles. These methods differ by their principles and the nature of drug molecules that could be encapsulated. Some successfully marketed products led to an enlargement of the applications and the interest given by researchers to these drug delivery systems. Choice of the technique and operating conditions is crucial to obtain formulations bearing good properties for in vitro and in vivo applications. In this review, we will focus on polymeric particles and give a scope about the most used polymers. We will also describe the common preformed polymer based techniques used for the encapsulation of drug molecules. We will also review the major applications of the developed particles during the last years and their main properties.
1. Polymer-solvent interactions
Many techniques that rely on preformed polymers have been used for the preparation of particulate carriers. Although these methods are quite different, they generally share a unique principle which is polymer precipitation. Precipitation of the polymer occurs either when a non solvent is added or after subsequent decrease of its solubility in a solvent. Many parameters could influence polymer solubility such as, solvent nature, pH, salinity and temperature of the dispersion medium. Solubility of polyelec-trolytes in water, for example, is highly pH and salinity dependent (Gennes, 1979[96]), while that of poly(alkyl acrylamide) and poly(alkyl methacrylamide), is mainly temperature dependent (Elaissari, 2002[77]). In fact, nanoprecipitation and emulsion based techniques are based on the addition of a non solvent to the polymer which causes its precipitation. However, ionic gelation technique, for instance, in which generally a polyelectrolyte is used as polymer, is based on the addition of a salt or an oppositely charged polymer. This results in a change in the salinity of the medium and the appearance of electrostatic interactions and thus, leads to polymer precipitation. The thermodynamic behavior of the polymer in a given solution is highly dependent on the Flory Χ-parameter. This parameter is defined as the free energy change per solvent molecule (in kBT units) when a solvent-solvent contact is shifted to a solvent-polymer contact. It is expressed by the following mathematical equations:
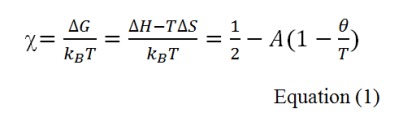
where kB and T are Boltzmann constant and temperature, respectively; A and Θ parameters are defined as follows:


It can be seen that the A parameter is directly related to entropy changes, whereas Θ temperature is a function of both entropic and enthalpic variations. When Θ temperature = T, the corresponding Flory Χ-parameter = 1/2, at which the second Virial coefficient is equal zero (Elias, 2003[79]). The latter can be easily determined from light scattering measurements of a diluted polymer solution. At Θ temperature conditions, the binary interactions among constituents will be negligible and only the excluded volume effects will be predominant. Consequently, the solvent will be a good solvent for the polymer when Χ < 1/2 and a poor one when Χ > 1/2 (Minost et al., 2012[178]).
2. Commonly used polymers for encapsulation
Several polymers have been used for drug encapsulation but only biodegradable and biocompatible ones are suitable for biomedical applications. The biodegradability of a polymer is acquired by the presence of a labile function such as ester, orthoester, anhydride, carbonate, amide, urea or urethane in their backbone. These polymers could be of natural (polysaccharides and protein based polymers) or synthetic (polyesters) nature (Pillai and Panchagnula, 2001[197]). The most commonly used polymers for drug encapsulation are polyesters (lactide and glycolide copolymers, poly-ε-caprolactone), acrylic polymers (polymethacrylates) and polyamides (gelatin and albumin). The selection of the right polymer is a crucial step to obtain particles that are suitable for a well-defined application. In fact, polymers’ structures are highly different and their surface and bulk properties are highly relevant for the obtaining of the desirable biological application. Copolymers could be also used to monitor the hydrophobicity of the materials. Some polymers are poly(ethyleneglycol) (PEG) copolymerized in order to decrease nanoparticle recognition by the reticular endothelial system. Table 1(Tab. 1) contains examples of the most used biocompatible and biodegradable polymers in encapsulation. Some polymers, especially those having mucoadhesive properties, could also be used for coating the nanocarriers (Mazzaferro et al., 2012[172]; Zandanel and Vauthier, 2012[273]).
Table 1. Commonly used polymers.
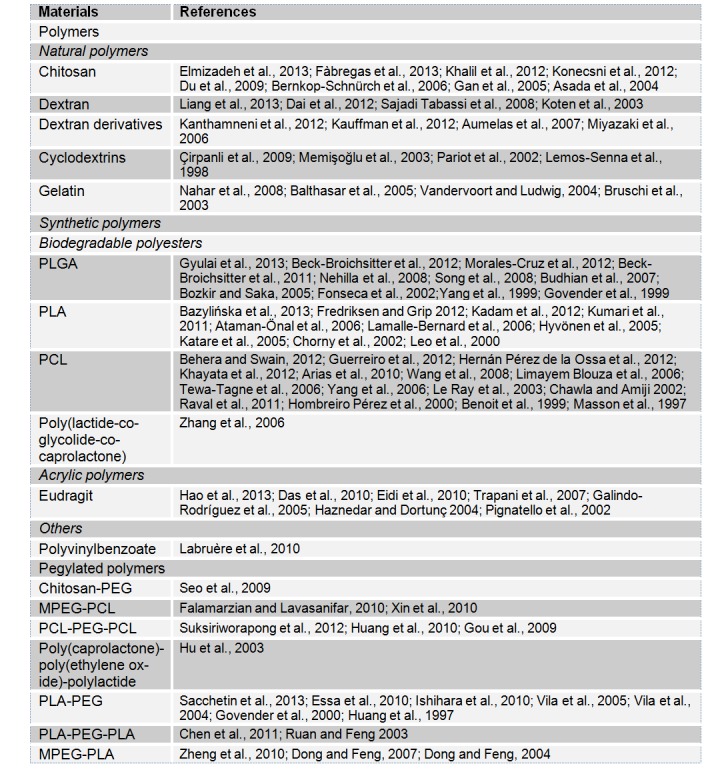
Table 1 (References listed)
Polymers
Natural polymers
Chitosan: Elmizadeh et al., 2013[81]; Fàbregas et al., 2013[85]; Khalil et al., 2012[141]; Konecsni et al., 2012[146]; Du et al., 2009[73]; Bernkop-Schnürch et al., 2006[28]; Gan et al., 2005[93]; Asada et al., 2004[12]
Dextran: Liang et al., 2013[161]; Dai et al., 2012[57]; Sajadi Tabassi et al., 2008[215]; Koten et al., 2003[147]
Dextran derivatives: Kanthamneni et al., 2012[132]; Kauffman et al., 2012[137]; Aumelas et al., 2007[15]; Miyazaki et al., 2006[179]
Cyclodextrins: Çirpanli et al., 2009[48]; Memisoglu et al., 2003[175]; Pariot et al., 2002[192]; Lemos-Senna et al., 1998[156]
Gelatin: Nahar et al., 2008[187]; Balthasar et al., 2005[18]; Vandervoort and Ludwig, 2004[250]; Bruschi et al., 2003[34]
Synthetic polymers
Biodegradable polyesters
PLGA: Gyulai et al., 2013[107]; Beck-Broichsitter et al., 2012[24]; Morales-Cruz et al., 2012[184]; Beck-Broichsitter et al., 2011[23]; Nehilla et al., 2008[188]; Song et al., 2008[232]; Budhian et al., 2007[35]; Bozkir and Saka, 2005[33]; Fonseca et al., 2002[89]; Yang et al., 1999[264]; Govender et al., 1999[102]
PLA: Bazylinska et al., 2013[22]; Fredriksen and Grip 2012[90]; Kadam et al., 2012[128]; Kumari et al., 2011[150]; Ataman-Önal et al., 2006[14]; Lamalle-Bernard et al., 2006[153]; Hyvönen et al., 2005[120]; Katare et al., 2005[136]; Chorny et al., 2002[46]; Leo et al., 2000[157]
PCL: Behera and Swain, 2012[26]; Guerreiro et al., 2012[104]; Hernán Pérez de la Ossa et al., 2012[115]; Khayata et al., 2012[143]; Arias et al., 2010[10]; Wang et al., 2008[258]; Limayem Blouza et al., 2006[162]; Tewa-Tagne et al., 2006[241]; Yang et al., 2006[263]; Le Ray et al., 2003[154]; Chawla and Amiji 2002[43]; Raval et al., 2011[209]; Hombreiro Pérez et al., 2000[116]; Benoit et al., 1999[27]; Masson et al., 1997[170]
Poly(lactide-co-glycolide-co-caprolactone): Zhang et al., 2006[277]
Acrylic polymers
Eudragit: Hao et al., 2013[110]; Das et al., 2010[60]; Eidi et al., 2010[76]; Trapani et al., 2007[243]; Galindo-Rodríguez et al., 2005[92]; Haznedar and Dortunç 2004[111]; Pignatello et al., 2002[196]
Others
Polyvinylbenzoate: Labruère et al., 2010[152]
Pegylated polymers
Chitosan-PEG: Seo et al., 2009[221]
MPEG-PCL: Falamarzian and Lavasanifar, 2010[86]; Xin et al., 2010[260]
PCL-PEG-PCL: Suksiriworapong et al., 2012[238]; Huang et al., 2010[118]; Gou et al., 2009[100]
Poly(caprolactone)-poly(ethylene oxide)-polylactide: Hu et al., 2003[117]
PLA-PEG: Sacchetin et al., 2013[214]; Essa et al., 2010[84]; Ishihara et al., 2010[123]; Vila et al., 2005[254]; Vila et al., 2004[253]; Govender et al., 2000[101]; Huang et al., 1997[119]
MPEG-PLA: Zheng et al., 2010[279]; Dong and Feng, 2007[70]; Dong and Feng, 2004[71]
2.1 Natural polymers
2.1.1 Chitosan
Chitosan is obtained by deacetylation of chitin, which is the structural element in the exoskeleton of crustaceans (crabs, shrimp, etc.) and cell walls of fungi. It is a cationic and biodegradable polysaccharide consisting of repeating D-glucosamine and N-acetyl-D-glucosamine units, linked via (1-4) glycosidic bonds. Chitosan is non toxic and can be digested in the physiological environment, either by lysozymes or by chitinases, which are present in the human intestine and in the blood. These properties led to increased interest for this polymer in pharmaceutical research and industry as a carrier for drug delivery (Mao et al., 2010[167]). In addition, chitosan has mucoadhesive properties owing to its positive charge that allows interaction with the negatively-charged mucosal surface. Consequently, the use of chitosan as a matrix (Patil and Sawant, 2011[194]) or as a coating material (Mazzarino et al., 2012[173]) in drug encapsulation had become a promising strategy to prolong the residence time, to increase the absorption of active molecules through the mucosa (Mao et al., 2010[167]; Alpar et al., 2005[7]) and also for targeted delivery (Park et al., 2010[193]).
2.1.2 Dextran and its derivatives
Dextran polymers are produced by bacteria from sucrose. Chemical synthesis is also possible. These glucose polymers consist predominantly of linear α-1,6-glucosidic linkage with some degree of branching via 1,3-linkage. Dextran-based microspheres have got much attention because of their low toxicity, good biocompatibility and biodegradability, which are of interest for application in biomedical and pharmaceutical fields (Mehvar, 2000). Many detxran polymers such as Sephadex® (cross-linked dextran microspheres) as well as Spherex® (cross-linked starch microspheres) were used as carriers for drug delivery. Other derivatives of dextran and starch including diethyl aminoethyl dextran and polyacryl starch have also been used for mucosal drug delivery. Illum et al. (2001[122]) proposed some mechanisms to explain absorption enhancement effects of cross-linked starch and dextran microspheres intended to nasal delivery which are: (1) Deposition of the microspheres in the less or non ciliated anterior part of the nasal cavity and slower nasal clearance; (2) Retention of the formulation in the nasal cavity for an extended time period because of the bioadhesive properties of the microspheres and (3) The local high drug concentration provided by the gelled system in close contact with the epithelial absorptive surface (Illum et al., 2001[122]).
2.1.3 Cyclodextrins
Cyclodextrins (CDs) are cyclic oligosaccharides that contain at least six D-(+) glucopyranose units which are attached by α-(1,4) glucosidic bonds. They have been widely used for the formulation of drugs with bioavailability concerns resulting from poor solubility, poor stability and severe side effects. There are 3 natural CDs which are α -, ß-, and γ-CDs (with 6, 7, or 8 glucose units respectively) (Challa et al., 2005[42]). In addition, amphiphilic cyclodextrins are synthetic derivatives of natural cyclodextrins. Such derivatives are able to self-organize in water to form micelles and nano-aggregates, which is interesting for pharmaceutical applications, mainly, encapsulation (Gèze et al., 2002[97]). In fact, amphiphilic cyclodextrins have recently been used to prepare nanoparticles and nanocapsules without surfactants and have shown high drug-loading capacity with favorable release properties (Lemos-Senna et al., 1998[156]; Çirpanli et al., 2009[48]; Duchêne, 1999[75]). They have also been used for targeting and for increasing drug loading (Duchêne et al., 1999[75]).
2.1.4 Gelatin
Gelatin is a natural polymer that is derived from collagen. It is commonly used for pharmaceutical and medical applications because of its biodegradability and biocompatibility in physiological environments. Gelatin is attractive for use in controlled release due to its nontoxic, bioactive properties and inexpensive price. It is also a polyampholyte having both cationic and anionic groups along with hydrophilic groups. Mechanical properties, swelling behavior and thermal properties of gelatin depend significantly on its crosslinking degree (Young et al., 2005[270]).
2.2 Biodegradable polyesters
Polyester-based polymers are among of the most widely investigated materials for drug delivery. Poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and their copolymers poly(lactic acid-co-glycolic acid) (PLGA) along with poly-ε-caprolactone are some of the well-defined biomaterials with regard to design and performance for drug-delivery applications.
2.2.1 PLGA
PLGA, a copolymer of lactic acid and glycolic acid, has generated tremendous interest due to its excellent biocompatibility, biodegradability, and mechanical strength. PLGA is approved by the US FDA and European Medicine Agency (EMA) in various drug delivery systems in humans. In order to improve the formulation of controlled drug delivery systems, an understanding of the physical, chemical, and biological properties of polymers is helpful. In fact, the polymer is commercially available with different molecular weights and copolymer compositions. The degradation time can vary from several months to several years, depending on the molecular weight and copolymer ratio (Danhier et al., 2012[59]). For example, lactic acid is more hydrophobic than glycolic acid and, therefore, lactide-rich PLGA copolymers are less hydrophilic, absorb less water, and subsequently, degrade more slowly (Dinarvand et al., 2011[68]). PLGA particles are widely used to encapsulate active molecules with a broad spectrum of pharmaceutical applications (Danhier et al., 2012[59]; Menei et al., 2005[176]; Singh et al., 2004[226]).
2.2.2 PLA
PLA is a biocompatible and biodegradable synthetic polyester which undergoes scission in the body to monomeric units of lactic acid. The latter is a natural intermediate in carbohydrate metabolism. PLA possess good mechanical properties and it is largely used for the preparation of particles (Gupta and Kumar, 2007[105]).
2.2.3 PCL
It was in 1930s that the ring-opening polymerization of PCL was studied. The biodegradable property of this synthetic polymer was first identified in 1973. PCL is suitable for controlled drug delivery due to its high permeability to many drugs and non-toxicity (Sinha et al., 2004[227]). Molecular weight dependent surface hydrophobicity and crystallinity of PCL are the causes for its slower biodegradation in two distinct phases such as random non-enzymatic cleavage and enzymatic fragmentation. Lipophilic drugs are generally distributed uniformly in the matrix while hydrophilic drugs tend to move towards the interface and remain on the surface of PCL formulation in adsorbed state. Diffusion was described as the only possible mechanism by which the lipophilic drugs release from PCL particles as they were shown to be intact for a much longer duration in vivo. However, two phenomena could be implicated in hydrophilic drugs’ release. Highly lipophilic drugs that resist complete diffusion are released upon surface erosion by enzymatic action while hydrophilic drugs that accumulate at the interface during the formulation processes are released by de-sorption at the initial period of release study or dosage intake. This results in a biphasic drug release pattern for PCL particles with much higher burst release for hydrophilic drugs than lipophilic ones (Dash and Konkimalla, 2012[61]).
2.3 Pegylated polymers
Many of the above cited polymers could be conjugated to PEG chains, which allows the enhancement of their hydrophilicity and permits the obtaining of a stealth surface that could protect the prepared carriers from degradation by the cells belonging to the reticuloendothelial system. Conjugation to PEG confers also bioadhesive properties for the carriers (Yoncheva et al., 2005[266]).
3. Used methods for the encapsulation of active molecules
3.1 Nanoprecipitation
The nanoprecipitation technique was first developed by Fessi et al. in 1986 (Devissaguet et al., 1991[66]). The technique allows the obtaining of either nanospheres or nanocapsules. The organic phase could be added to the aqueous phase under magnetic stirring. This one-step process allows the instantaneous and reproducible formation of monodisperse nanoparticles. Nanoprecipitation is simple, is by far the fastest, most reproducible, and industrially feasible preparation procedure of nanospheres (Vauthier and Bouchemal, 2009[251]). Practically, two miscible phases are required: an organic solvent in which the polymer is dissolved and an aqueous phase (non-solvent of the polymer). The most common used organic solvents are ethanol and acetone. Such solvents are miscible in water and easy to remove by evaporation. Some oils could be added to these solvents to allow the dissolving of the active (Rosset et al., 2012[212]). As Figure 1(Fig. 1) (Pinto Reis et al., 2006[198]) shows, the method is based on the addition of one phase to the other under moderate magnetic stirring which causes the interfacial deposition of a polymer after displacement of the organic solvent from the organic solution. This leads to the formation of a suspension of nanoparticles. The organic phase could be a mixture of solvents such as, mixture of acetone with water or ethanol etc. Similarly, the aqueous phase could consist of a mixture of non-solvents and could contain surfactants. Commonly used polymers are biodegradable polyesters, especially PCL, PLA and PLGA (Rao and Geckeler, 2011[207]). Particle formation process includes three basic steps which are, particle nucleation, molecular growth and aggregation. The rate of every step has a crucial impact on the particle size distribution. Supersaturation is the driving force that manages all of these steps, namely, particles nucleation rate. Supersaturation, itself, is influenced by fluid dynamics and mixing. In fact, low stirring rate results in low nucleation rates while higher mixing rates give high nucleation rates (Lince et al., 2008[163]).
Figure 1. The nanoprecipitation technique (Pinto Reis et al., 2006).
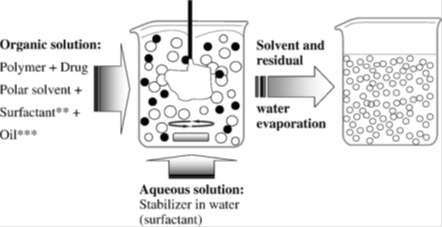
Operational parameters that should be controlled include the organic phase to non organic phase ratio, the concentration of the polymer and the stabilizer and the amount of the drug. Every one of these parameters may exert an impact on the characteristics of the obtained nanoparticles (size, uniformity and charge). In fact, an increase of the polymer amount generally increases particles’ size (Chorny et al., 2002[46]; Simsek et al., 2013[225]; Dong and Feng, 2004[71]; Asadi et al., 2011[13]). The same effect was obtained after increasing the polymer molecular weight (Limayem Blouza et al., 2006[162]; Holgado et al., 2012). These findings were explained by an increase of the viscosity of the organic phase which rendered solvent diffusion more difficult and thus, led to larger nanoparticles’ size. The effect of increasing organic phase volume seems conflicting: some studies showed that it causes a decrease of the particles size (Dong and Feng, 2004[71]) while others showed the opposite phenomenon (Asadi et al., 2011[13]). Increasing the water phase amount leads to a decrease of the particles size as a result of the increased diffusion of the water-miscible solvent in the aqueous phase and thus, the more rapid precipitation of the polymer and formation of nanoparticles (Budhian et al., 2007[35]). An increase of the surfactant amount generally causes a decrease of the particles size and reduces size distribution (Contado et al., 2013[50]; Siqueira-Moura et al., 2013[228]). Some studies did not, however, found significant change following surfactant amount increase (Dong and Feng, 2004[71]). The nature of the surfactant may also influence the particles’ size (Limayem Blouza et al., 2006[162]). Increasing mixing rate decreases the particles size as it causes faster diffusion rate (Asadi et al., 2011[13]). Theoretical drug loading may also influence particles size and drug loading (Govender et al., 1999[102]). Nanoprecipitation is generally designed for the encapsulation of hydrophobic drug molecules (Seju et al., 2011[220]; Katara and Majumdar, 2013[135]; Seremeta et al., 2013[222]). Such actives may be dissolved within the organic phase. Bilalti et al. (2005[29]) described a nanoprecipitation technique intended to the encapsulation of hydrophilic molecules but the size of the obtained particles was not sufficiently uniform (Bilati et al., 2005[29]). To further improve the reproducibility of the nanoprecipitation technique and make it more convenient for industrial applications, membrane contactor and microfluidic technology were successfully used (Khayata et al., 2012[143]; Xie and Smith, 2010[259]). These techniques allow better size control within different batches of particles. Table 2(Tab. 2) contains some examples of the applications of the nanoprecipitation technique in drug delivery during the last years. It can be concluded that polyesters are among the most used polymers for the preparation of the nanoparticles by this technique.
Table 2. Applications of the nanoprecipitation technique.
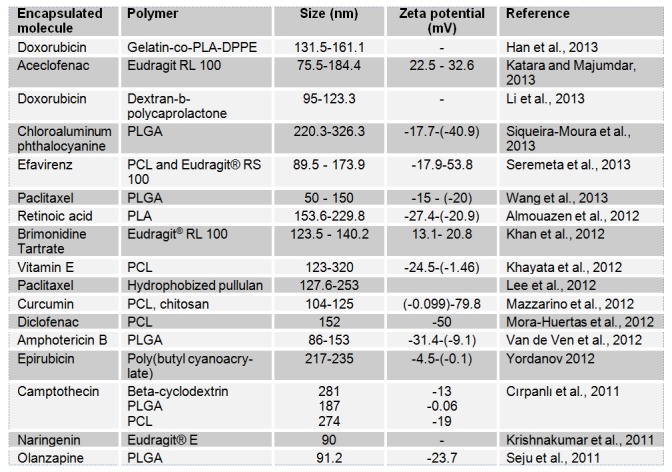
Table 2 (References listed)
Doxorubicin: Han et al., 2013[109]
Aceclofenac: Katara and Majumdar, 2013[135]
Doxorubicin: Li et al., 2013[158]
Chloroaluminum phthalocyanine: Siqueira-Moura et al., 2013[228]
Efavirenz: Seremeta et al., 2013[222]
Paclitaxel: Wang et al., 2013[257]
Retinoic acid: Almouazen et al., 2012[6]
Brimonidine Tartrate: Khan et al., 2012[142]
Vitamin E: Khayata et al., 2012[143]
Paclitaxel: Lee et al., 2012[155]
Curcumin: Mazzarino et al., 2012[173]
Diclofenac: Mora-Huertas et al., 2012[183]
Amphotericin: Van de Ven et al., 2012[246]
Epirubicin: Yordanov, 2012[268]
Camptothecin: Cirpanli et al., 2011[47]
Naringenin: Krishnakumar et al., 2011[148]
Olanzapine: Seju et al., 2011[220]
3.2 Emulsion diffusion (ESD)
ESD was first developed by Quintanar-Guerrero and Fessi (Quintanar-Guerrero et al., 1996[204]) to prepare PLA based nanospheres. Three liquid phases are needed in this technique: an organic phase, an aqueous phase and a dilution phase. The organic phase generally contains the polymer and the hydrophobic drug. The aqueous phase is a solution of a stabilizing agent while the dilution phase usually consists of a large volume of water. Mutual saturation of the aqueous and organic phase allows further obtaining of a thermodynamically equilibrated emulsion upon high speed homogenization. Subsequent addition of an excess of water enables the diffusion of the organic solvent from the dispersed phase resulting in precipitation of the polymer and the formation of the particles (Figure 2(Fig. 2); Pinto Reis et al., 2006[198]). Commonly used polymers in this method include PCL, PLA and Eudragit® (Mora-Huertas et al., 2010[182]). Table 3(Tab. 3) shows that the technique is mainly used for the encapsulation of hydrophobic molecules. However, hydrophilic molecules may also be encapsulated by a modified solvent diffusion method using an aqueous inner phase (Ma et al., 2001[164]). Operating conditions affecting the size of the obtained particles include external/internal phase ratio, emulsification stirring rate, volume and temperature of water for dilution, polymer amount and concentration of the stabilizer (Quintanar-Guerrero et al., 1996[204]; Mora-Huertas et al., 2010[182]). Influence of high shear homogenization and sonication on the particles size was assessed and it was found that sonication was more efficient for particle size reduction. The nature of the surfactant influenced also the particles size. In fact, when Pluronic F68 (PF68), didodecyldimethylammonium bromide (DMAB) and polyvinylalcohol (PVA) were compared, DMAB gave the smallest particles but with the lowest encapsulation efficiency (Jain et al., 2011[125]). Particles size was also described to increase with an increase of initial drug amount (Youm et al., 2012[269]), polymer amount (Youm et al., 2012[269]; Esmaeili et al., 2011[82]) and the oil phase volume (Esmaeili et al., 2011[82]; Poletto et al., 2008[199]). An increase of the surfactant amount resulted in a decrease of the size but it seems that above some level further significant size reduction is no longer possible (Jain et al., 2011[125]; Surassmo et al., 2010[239]). An increase of the homogenization rate led to a decrease of the particles’ size (Jain et al., 2011[125]; Kwon et al., 2001[151]; Galindo-Rodríguez et al., 2005[92]). Likely, the same effect was obtained following an increase of the temperature and the volume of added water (Kwon et al., 2001[151]; Song et al., 2006[231]). The nature of the organic solvent also influenced particle size (Song et al., 2006[231]). Table 3 shows some of the recent applications of the ESD technique.
Figure 2. Emulsion diffusion technique (Pinto Reis et al., 2006).
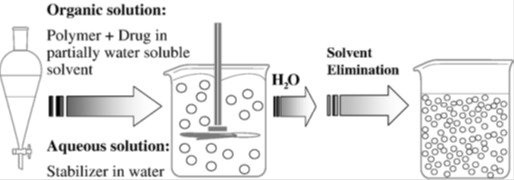
Table 3. Applications of the emulsion diffusion method.
Table 3 (References listed)
Articaine: Campos et al., 2013[39]
Omeprazole: Hao et al., 2013[110]
Curcumin: Souguir et al., 2013[234]
Matricaria recutita L. extract: Esmaeili et al., 2011[82]
Bovine serum albumin: Karnchanajindanun et al., 2011[134]
Alendronate: Cohen-Sela et al., 2009[49]
An oligonucleotide: Delie et al., 2001[64]
3.3 Simple emulsion evaporation (SEE)
The SEE technique is widely used in the field of particulate carriers’ development. This method was first developed by (Vanderhoff et al., 1979[249]). It consists on the formation of a simple emulsion followed by the evaporation of the organic solvent. Subsequent precipitation of the polymer allows the obtaining of the particles (Figure 3(Fig. 3), Pinto Reis et al., 2006[198]). Practically, for oil in water emulsion method, the polymer is dissolved in a volatile and non miscible organic solvent such as chloroform, ethylacetate or dichloromethane. This organic phase, in which the drug and the polymer are dissolved, is then dispersed by high speed homogenization or by sonication in an aqueous phase containing a surfactant. Once an oil-in-water (o/w) emulsion is obtained, the evaporation of the organic solvent permits the precipitation of the polymer and thus, the formation of the particles. As it can be seen in Table 4, SEE is generally used for the encapsulation of hydrophobic drugs (O’Donnell and McGinity, 1997[189]). The evaporation of the organic solvent is obtained by moderately stirring the emulsion at room temperature or under high temperature and low pressure conditions. The obtained particles can be then harvested by ultracentrifugation or filtration, then washed and lyophilized. Membrane technology was also used to prepare particles by the simple emulsion technique (Doan et al., 2011[69]). Another alternative of the technique is the use of water in oil emulsion method that is suitable for the encapsulation of hydrophilic active molecules. Particulate carriers are obtained after evaporation of the water phase which causes the precipitation of the hydrophilic polymer (Banerjee et al., 2012[19]). Parameters that have to be managed include organic phase to water phase ratio, nature of the surfactant and its concentration, stirring rate, polymer amount and evaporation rate. Decreasing the organic solvent volume resulted generally in a decrease of particle size (Budhian et al., 2007[35]). Particle size could also be decreased by increasing surfactant amount (Valot et al., 2009[245]; Manchanda et al., 2010[166]; Khaled et al., 2010[139]; Su et al., 2009[236]), increasing stirring rate (Su et al., 2009[236]; Lee et al., 2012[155]; Avachat et al., 2011[16]; Yadav and Sawant, 2010[261]) or increasing aqueous phase volume (Adibkia et al., 2011[1]). However, an increase of polymer amount generally increases particles’ size (Doan et al., 2011[69]; D’Aurizio et al., 201[55]1; Adibkia et al., 2011[1]; Agnihotri and Vavia, 2009[2]). Table 4(Tab. 4) shows the applications of the SEE technique in drug delivery. Polyesters were widely used for the encapsulation of hydrophobic drugs.
Figure 3. Simple emulsion solvent evaporation (Pinto Reis et al., 2006).
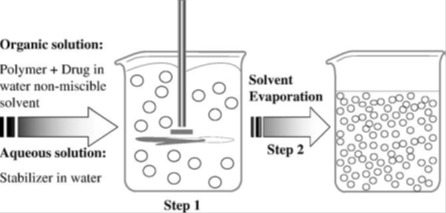
Table 4. Applications of simple emulsion solvent evaporation technique.
Table 4 (References listed)
Curcumin: Khalil et al., 2013[140]
Efavirenz: Seremeta et al., 2013[222]
Human amylin: Guerreiro et al., 2012[104]
Azithromycin: Li et al., 2012[160]
Teniposide: Mo et al., 2012[180]
Camptothecin: Dai et al., 2011[58]
Naproxen: Javadzadeh et al., 2010[126]
Doxorubicin: Manchanda et al., 2010[166]
Dexamethasone: Rawat and Burgess, 2010[210]
Ibuprofen: Valot et al., 2009[245]
3.4 Double emulsion evaporation (DEE)
Double emulsion technique is suitable for the encapsulation of hydrophilic molecules (see Table 5(Tab. 5) and Figure 4(Fig. 4); Giri et al., 2013[98]). Generally, the method consists on the dispersion of an aqueous phase in a non miscible organic solvent to form the first emulsion (W1/O). This dispersion is performed under high shear homogenization or low power sonication for a short time. This step is followed by the dispersion of the obtained emulsion in a second aqueous phase containing a hydrophilic emulsifier. Again, homogenization could be carried under high shear homogenization or with a sonication probe. When sonication is used, it must be performed at low power and within a short period of time to not break the first emulsion (Giri et al., 2013[98]). After the formation of the multiple emulsion, evaporation of the volatile organic solvent under low pressure (by a rotary evaporator) or at ambient temperature allows the obtaining of the particulate carriers (Figure 4(Fig. 4); Giri et al., 2013[98]). There are other types of multiple emulsions like w/o/o or o/w/o (Giri et al., 2013[98]). A lot of parameters may influence the properties of the obtained particles such as, relative phases’ ratio (Khoee et al., 2012[144]), amount of the polymer, its nature and molecular weight (Zambaux et al., 1998[272]; Péan et al., 1998[195]; Van de Ven et al., 2011[247]), nature of the surfactants and their amounts (Zhao et al., 2007[278]; Khoee and Yaghoobian, 2009[145]; Dhanaraju et al., 2004[67]), homogenization speed (Eley and Mathew, 2007[78]; Basarkar et al., 2007[21]), the composition of the external phase (Péan et al., 1998[195]; Tse et al., 2009[244]) and evaporation speed (Khoee et al., 2012[144]). Operating conditions may also influence strongly encapsulation efficiency (Tse et al., 2009[244]; Billon et al., 2005[30]; Silva et al., 2013[224]; Zhou et al., 2013[280]; Karatas et al., 2009[133]; Hachicha et al., 2006[108]; Al-Kassas, 2004[5]; Cun et al., 2011[54]; Gaignaux et al., 2012[91]; Cun et al., 2010[53]). Membrane technique and microfluidic devices were also used to prepare particulate carriers by the DES method (Vladisavljevic and Williams, 2008[255]; van der Graaf et al., 2005[248]).
Table 5. Applications of the double emulsion technique.
Figure 4. Double emulsion solvent evaporation technique (Giri et al., 2013).
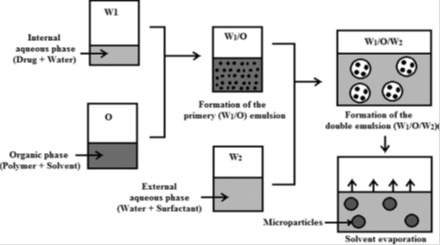
Table 5 (References listed)
3.5 Spray drying
Spray drying is a simple process which gained too much interest due to its cost-effectiveness and scalability (Sou et al., 2011[233]). Practically, a polymer containing drug solution is atomized and sprayed into a drying chamber where droplets are dried by heated air (See Figure 5(Fig. 5); Pinto Reis et al., 2006[198]). Reduction of droplets’ size that follows atomization allows the obtaining of an enormous surface area between droplets and the drying gas. The subsequent precipitation of the polymer permits the encapsulation of the drug within the obtained particulate carriers. The evaporation of the solvent occurs within a very short period of time. Consequently, the materials never reach the inlet temperature of drying gas. This is very attractive for encapsulating heat-sensitive drug molecules like proteins (Cal and Sollohub, 2010[38]; Sollohub and Cal, 2010[230]; Prata et al., 2013[201]). Many operating conditions could influence the properties of the obtained particles. Parameters to be controlled include the drying air temperature and humidity (Bruschi et al., 2003[34]), the rate and fluid dynamics of the air flow, the atomization process (Droplet size, spray rate, spray mechanism) and the composition of ingredients and excipients in the feeding solution (Rattes and Oliveira, 2007[208]). PLA (Baras et al., 2000[20]; Gander et al., 1996[94]; Sastre et al., 2007[219]; Muttil et al., 2007[186]), PLGA (Wang and Wang, 2002[256]; Mu and Feng, 2001[185]; Castelli et al., 1998[40]; Bittner et al., 1999[31]; Prior et al., 2000[202]; Conti et al., 1997[51]), PCL, methacrylate polymers (Esposito et al., 2002[83]; Año et al., 2011[8]; Cruz et al., 2010[52]; Hegazy et al., 2002[114]; Raffin et al., 2008[206]) and chitosan (He et al., 1999[113]; Giunchedi et al., 2002[99]; Cevher et al., 2006[41]) are among the most used polymers in spray-drying method. As Table 6(Tab. 6) shows, the technique allowed the obtaining of mainly microparticles bearing better drug solubility and sustained release.
Figure 5. The spray drying method (Pinto Reis et al., 2006).
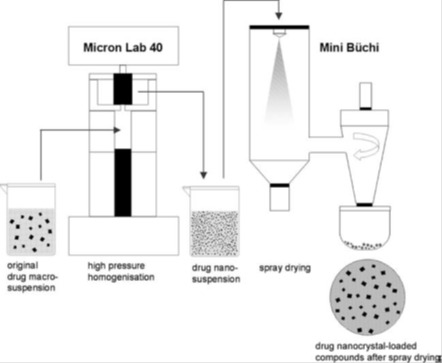
Table 6. Applications of the spray drying technique.
Table 6 (References listed)
Nimodipine: Bege et al., 2013[25]
Theophylline: Garekani et al., 2013[95]
Ofloxacin: Palazzo et al., 2013[191]
Sodium diclofenac: Tawfeek, 2013[240]
Sodium fluoride: Keegan et al., 2012[138]
Plasmid: Mohajel et al., 2012[181]
Heparin: Yildiz et al., 2012[265]
Alendronate: Cruz et al., 2010[52]
Zolmitriptan: Alhalaweh et al., 2009[4]
Triamcinolone: da Silva et al., 2009[56]
Acyclovir: Stulzer et al., 2009[235]
3.6 Supercritical fluid technology (SFT)
In the recent years, novel particle formation techniques using supercritical fluids (SCF) have been developed in order to overcome some of the disadvantages of conventional techniques that are: (1) poor control of particle size and morphology; (2) degradation and lost of biological activity of thermosensitive compounds; (3) low encapsulation efficiency and (4) low precipitation yield (Santos et al., 2013[218]). Moreover, SFT presents the main advantage of not requiring the use of toxic solvents. In fact, SCF based technologies have attracted enormous interest for the production of microparticles and nanoparticles (Table 7), since their emergence in the early 1990s (Sanli et al., 2012[217]).
The supercritical state is achieved when a substance is exposed to conditions above its critical pressure and temperature. In such conditions, the fluid will have liquid-like density and, thus, solvating properties that are similar to those of liquids and, at the same time, gaslike mass transfer properties. Carbon dioxide (CO2) is the most commonly used critical fluid. In fact, CO2 is nontoxic, nonflammable and easy recyclable. Moreover, CO2 has moderate critical parameters of CO2 (a critical pressure of 7.4 MPa and a critical temperature of 304.1 K) and low price and is highly available which makes it very attractive from an economical point of view and also for the processing of labile compounds (Elizondo et al., 2012[80]). Supercritical fluid technology methods can be divided in four methods which are rapid expansion of supercritical solution (RESS), Particles from gas saturated solutions (PGSS), gas antisolvent (GAS) and supercritical antisolvent process (SAS). These methods depend on whether CO2 was used as a solvent, a solute or an antisolvent. Figure 6(Fig. 6) (Byrappa et al., 2008[36]) shows the experimental set up of the RESS technique. In the RESS technique, the drug and the polymer are first dissolved in supercritical CO2 in high pressure chamber. The subsequent passing of the solution through a nozzle results in a rapid decrease of the pressure and thus, a precipitation of the drug particles embedded in the polymer matrix and their recovery in the extraction unit (Byrappa et al., 2008[36]). Many parameters such as the density of the SCF (Pressure and temperature of supercritical fluid) (Kalani and Yunus, 2012[129]), flow rate of drug-polymer solution and/or CO2 and formulation variables (Martin et al., 2002[168]) could influence the size of the obtained particles. Table 7(Tab. 7) shows that SFT was used for the processing of nanoparticles and microparticles mainly based on polyesters.
Figure 6. Schematic presentation of the experimental set up for the RESS process (Byrappa et al., 2008).
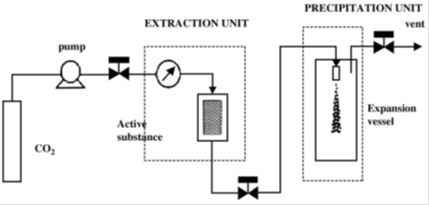
Table 7. Applications of the SCF technology.
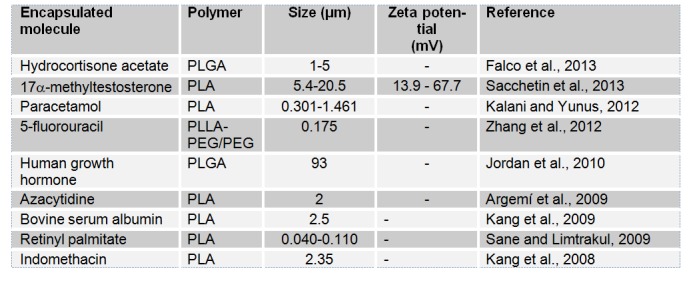
Table 7 (References listed)
Hydrocortisone acetate: Falco et al., 2013[87]
17α-methyltestosterone: Sacchetin et al., 2013[214]
Paracetamol: Kalani and Yunus, 2012[129]
5-fluorouracil: Zhang et al., 2012[274]
Human growth hormone: Jordan et al., 2010[127]
Azacytidine: Argemí et al., 2009[9]
Bovine serum albumin: Kang et al., 2009[131]
Retinyl palmitate: Sane and Limtrakul, 2009[216]
Indomethacin: Kang et al., 2008[130]
3.7 Ionic gelation (IG)
IG method is used mainly with natural hydrophilic polymers to prepare particulate carriers. These polymers include gelatin, alginate, chitosan and agarose. IG has the advantage of not using organic solvents. The technique is based on the transition of the polymer from liquid state to a gel (Figure 7(Fig. 7); Guenet, 1992[103]). For instance, gelatin based particles are obtained after the hardening of the droplets of emulsified gelatin solution. The particles are obtained after cooling gelatin emulsion droplets below the gelation point in an ice bath. For alginate, however, particles are produced by drop-by-drop extrusion of the sodium alginate solution into the calcium chloride solution. Sodium alginate is, in fact, a water-soluble polymer that gels in the presence of multivalent cations such as calcium. Chitosan particles are prepared by spontaneous formation of complexes between the positively charged chitosan and polyanions (tripolyphosphate or gelatin) or by the gelation of a chitosan solution dispersed in an oil emulsion (Mahapatro and Singh, 2011[165]). Figure 7(Fig. 7) (Guenet, 1992[103]) illustrates the gelation mechanism of polysaccharides. At high temperatures, a random coil conformation is assumed. With decreasing temperature, the aggregation of double helices structure forms the physical junctions of gels (Rees and Welsh, 1977[211]). Table 8(Tab. 8) displays some recent applications of IG. This technique has been mainly used to prepare chitosan nanoparticles.
Figure 7. Gelation mechanism of polysaccharides in water (Guenet, 1992).
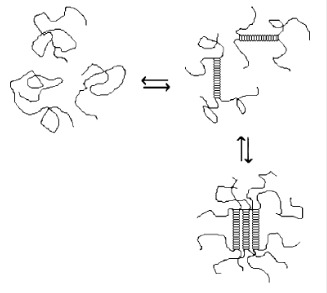
Table 8. Some applications of the ionic gelation technique.
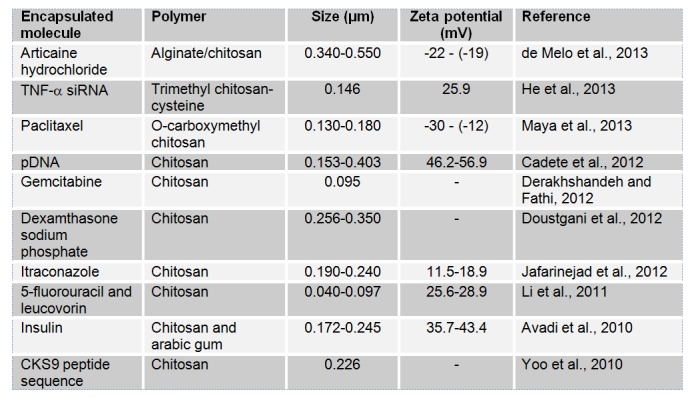
Table 8 (References listed)
Articaine hydrochloride: de Melo et al., 2013[62]
TNF-α siRNA: He et al., 2013[112]
Paclitaxel: Maya et al., 2013[171]
pDNA: Cadete et al., 2012[37]
Gemcitabine: Derakhshandeh and Fathi, 2012[64]
Dexamthasone sodium phosphate: Doustgani et al., 2012[72]
Itraconazole: Jafarinejad et al., 2012[124]
5-fluorouracil and leucovorin: Li et al., 2011[159]
Insulin: Avadi et al., 2010[17]
CKS9 peptide sequence: Yoo et al., 2010[267]
3.8 Layer by layer
Polyelectrolyte self assembly is also called layer-by-layer (LbL) assembly process. The earliest technology was based on the assembly of colloidal particles on a solid core (Iler, 1966[121]). From the 1990s, applications were expanded. LbL allowed, in fact, the assembly of polyelectrolyte films using biopolymers, proteins, peptides, polysaccharides and DNA (Powell et al., 2011[200]). This approach was first developed by Sukhorukov et al. (Sukhorukov et al., 1998[237]). Polyelectrolytes are classified according to their origin. Standard synthetic polyelectrolytes include poly(styrene sulfonate) (PSS), poly (dimethyldiallylammonium chloride) (PDDA), poly(ethylenimine) (PEI), poly(N-isopropyl acrylamide (PNIPAM), poly-(acrylic acid) (PAA), poly (methacrylic acid) (PMA), poly(vinyl sulfate) (PVS) and poly(allylamine) (PAH). Natural polyelectrolytes include nucleic acids, proteins and polysaccharides such as, alginic acid, chondroitin sulfate, DNA, heparin, chitosan, cellulose sulfate, dextran sulfate and carboxymethylcellulose (de Villiers et al., 2011[63]). The obtained particles are vesicular and are called polyelectrolyte capsules. Assembly process is based on irreversible electrostatic attraction that leads to polyelectrolyte adsorption at supersaturating polyelectrolyte concentrations. Other interactions such as, hydrogen bonds, hydrophobic interactions and Van der Waals forces were also described (de Villiers et al., 2011[63]). A colloidal template that serves to the adsorption of the polyelectrolyte is also needed. The commonly used cores for the formulated particles are derived from stabilized colloidal dispersions of charged silica, charged poly(styrene) spheres, metal oxides, polyoxometalates and conducting liquid crystalline polymers. Carrier systems can be functionalized with stimuliresponsive components that respond to temperature, pH and ionic strength. The polymers/colloids used in LbL technique can also be functionalized to alter their properties preceding layer by layer assembly. Experimental parameters that have to be managed include coating material concentration, ion concentration and the pH of the medium (Vergaro et al., 2011[252]). Polymer assembly occurs after incubation of the template in the polymer solution or by decreasing polymer solubility by drop-wise addition of a miscible solvent (Radtchenko et al., 2002[205]). This procedure could be repeated with a second polymer to allow sequential deposition of multiple polymer layers (Figure 8(Fig. 8); Ariga et al., 2011[11]). LbL presents advantages over several conventional coating methods: (1) simplicity of the process and equipment; (2) its suitability for coating most surfaces; (3) availability and abundance of natural and synthetic colloids; (4) flexible application to objects with irregular shapes and sizes; (5) formation of stabilizing coats and (6) control over the required multilayer thickness (de Villiers et al., 2011[63]). Table 9(Tab. 9) contains some recent applications of LbL technique.
Figure 8. The layer-by-layer technique based on electrostatic interaction (Ariga et al., 2011).
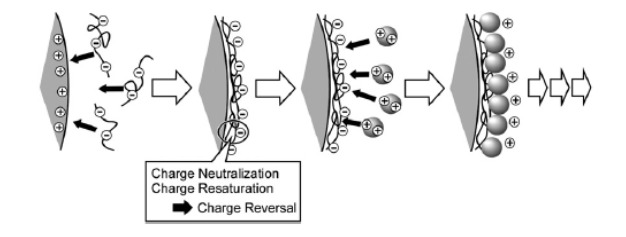
Table 9. Applications of the layer-by-layer technique.
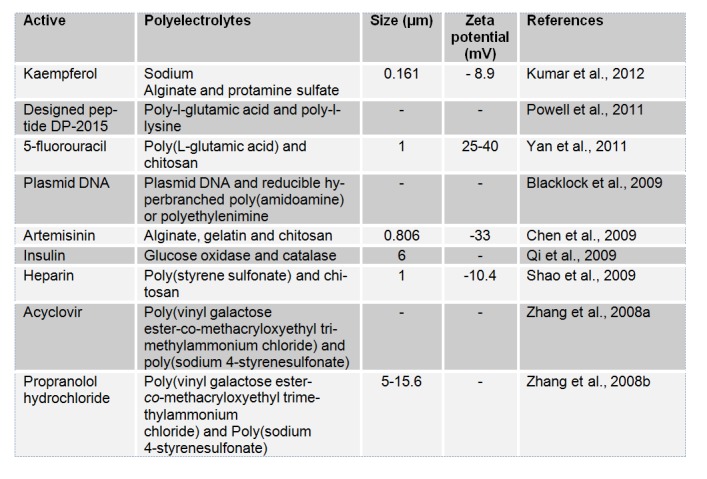
Table 9 (References listed)
Kaempferol: Kumar et al., 2012[149]
Designed peptide DP-2015: Powell et al., 2011[200]
5-fluorouracil: Yan et al., 2011[262]
Plasmid DNA: Blacklock et al., 2009[32]
Artemisinin: Chen et al., 2009[45]
Insulin: Qi et al., 2009[203]
Heparin: Shao et al., 2009[223]
Acyclovir: Zhang et al., 2008[275]
Propranolol hydrochloride: Zhang et al., 2008[276]
Conclusion
Encapsulation of active molecules is a crucial approach that has been widely used for many biomedical applications. It permits enhancement of bioavailability of molecules, sustained delivery, passive or active targeting and decrease of toxicity and side effects. These formulations can render some active molecules more suitable for a specific route such as the delivery of proteins by the oral route or the delivery of some drugs via the blood brain barrier. Thus, they enhance efficiency, patient compliance and allow successful management of diseases. Many biodegradable and biocompatible polymers were investigated. The choice of the technique and the suitable polymer is a crucial step. It depends on the physicochemical properties of the drug to be encapsulated. The management of operating conditions is also a hard task to monitor particles’ properties and to enhance drug loading. Recent research works are focusing on active targeting by the coating the carriers by biomolecules that specifically recognize a well-defined cell receptor. One can also notice a shift for more ’intelligent’ drug delivery systems. Responsive materials, for example, react to a specific physiological stimulus such as a variation of pH to release the encapsulated drug. Other thermosensitive materials deliver drugs at a specific temperature. It can be noted also that more attention is paid to safer methods that avoid the use of organic solvents (RESS) or to techniques that provide better reproducibility and easy scalability (micro-fluidics and membrane emulsification technology), which could be attractive for industrial processing.
References
- 1.Adibkia K, Javadzadeh Y, Dastmalchi S, Mohammadi G, Niri FK, Alaei-Beirami M. Naproxen-eudragit rs100 nanoparticles: preparation and physicochemical characterization. Colloids Surf B Biointerfaces. 2011;83:155–9. doi: 10.1016/j.colsurfb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Agnihotri SM, Vavia PR. Drug loaded polylac(glc-leu) microparticles: formulation and release characteristics. Colloids Surf B Biointerfaces. 2009;74:336–9. doi: 10.1016/j.colsurfb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad H. A special issue on polymer and hybrid particles for biomedical applications. J Colloid Sci Biotechnol. 2013;2:153–4. [Google Scholar]
- 4.Alhalaweh A, Andersson S, Velaga SP. Preparation of zolmitriptan-chitosan microparticles by spray drying for nasal delivery. Eur J Pharm Sci. 2009;38:206–14. doi: 10.1016/j.ejps.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Al-Kassas R. Design and in vitro evaluation of gentamicin-eudragit microspheres intended for intra-ocular administration. J Microencapsul. 2004;21:71–81. doi: 10.1080/02652040310001619992. [DOI] [PubMed] [Google Scholar]
- 6.Almouazen E, Bourgeois S, Boussaïd A, Valot P, Malleval C, et al. Development of a nanoparticle-based system for the delivery of retinoic acid into macrophages. Int J Pharm. 2012;430:207–15. doi: 10.1016/j.ijpharm.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Alpar HO, Somavarapu S, Atuah KN, Bramwell VW. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and dna delivery. Adv Drug Deliv Rev. 2005;57:411–30. doi: 10.1016/j.addr.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Año G, Esquisabel A, Pastor M, Talavera A, Cedré B, et al. A new oral vaccine candidate based on the microencapsulation by spray-drying of inactivated vibrio cholerae. Vaccine. 2011;29:5758–64. doi: 10.1016/j.vaccine.2011.05.098. [DOI] [PubMed] [Google Scholar]
- 9.Argemí A, Vega A, Subra-Paternault P, Saurina J. Characterization of azacytidine/poly(l-lactic) acid particles prepared by supercritical antisolvent precipitation. J Pharm Biomed Anal. 2009;50:847–52. doi: 10.1016/j.jpba.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Arias JL, López-Viota M, Sáez-Fernández E, Ruiz MA. Formulation and physicochemical characterization of poly(ɛ-caprolactone) nanoparticles loaded with ftorafur and diclofenac sodium. Colloids Surf B Biointerfaces. 2010;75:204–8. doi: 10.1016/j.colsurfb.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 11.Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. Layer-by-layer self-assembled shells for drug delivery. Adv Drug Deliv Rev. 2011;63:762–71. doi: 10.1016/j.addr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Asada M, Takahashi H, Okamoto H, Tanino H, Danjo K. Theophylline particle design using chitosan by the spray drying. Int J Pharm. 2004;270:167–74. doi: 10.1016/j.ijpharm.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Asadi H, Rostamizadeh K, Salari D, Hamidi M. Preparation of biodegradable nanoparticles of tri-block pla-peg-pla copolymer and determination of factors controlling the particle size using artificial neural network. J Microencapsul. 2011;28:406–16. doi: 10.3109/02652048.2011.576784. [DOI] [PubMed] [Google Scholar]
- 14.Ataman-Önal Y, Munier S, Ganée A, Terrat C, Durand P-Y, et al. Surfactant-free anionic pla nanoparticles coated with hiv-1 p24 protein induced enhanced cellular and humoral immune responses in various animal models. J Control Release. 2006;112:175–85. doi: 10.1016/j.jconrel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Aumelas A, Serrero A, Durand A, Dellacherie E, Leonard M. Nanoparticles of hydrophobically modified dextrans as potential drug carrier systems. Colloids Surf B Biointerfaces. 2007;59:74–80. doi: 10.1016/j.colsurfb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Avachat AM, Bornare PN, Dash RR. Sustained release microspheres of ropinirole hydrochloride: effect of process parameters. Acta Pharm Zagreb Croat. 2011;61:363–76. doi: 10.2478/v10007-011-0032-4. [DOI] [PubMed] [Google Scholar]
- 17.Avadi MR, Sadeghi AMM, Mohammadpour N, Abedin S, Atyabi F, et al. Preparation and characterization of insulin nanoparticles using chitosan and arabic gum with ionic gelation method. Nanomed Nanotechnol Biol Med. 2010;6:58–63. doi: 10.1016/j.nano.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Balthasar S, Michaelis K, Dinauer N, von Briesen H, Kreuter J, Langer K. Preparation and characterisation of antibody modified gelatin nanoparticles as drug carrier system for uptake in lymphocytes. Biomaterials. 2005;26:2723–32. doi: 10.1016/j.biomaterials.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee P, Deb J, Roy A, Ghosh A, Chakraborty P. Fabrication and development of pectin microsphere of metformin hydrochloride. ISRN Pharm. 2012:230621. doi: 10.5402/2012/230621.. Available from: http://dx.doi.org/10.5402/2012/230621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baras B, Benoit M-A, Gillard J. Parameters influencing the antigen release from spray-dried poly(dl-lactide) microparticles. Int J Pharm. 2000;200:133–45. doi: 10.1016/s0378-5173(00)00363-x. [DOI] [PubMed] [Google Scholar]
- 21.Basarkar A, Devineni D, Palaniappan R, Singh J. Preparation, characterization, cytotoxicity and transfection efficiency of poly(dl-lactide-co-glycolide) and poly(dl-lactic acid) cationic nanoparticles for controlled delivery of plasmid dna. Int J Pharm. 2007;343:247–54. doi: 10.1016/j.ijpharm.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazylińska U, Lewińska A, Lamch Ł, Wilk KA. Polymeric nanocapsules and nanospheres for encapsulation and long sustained release of hydrophobic cyanine-type photosensitizer. Colloids Surf Physicochem Eng Asp. Colloids Surf Physicochem Eng Asp. 2013 [Google Scholar]
- 23.Beck-Broichsitter M, Ruppert C, Schmehl T, Guenther A, Betz T, et al. Biophysical investigation of pulmonary surfactant surface properties upon contact with polymeric nanoparticles in vitro. Nanomed Nanotechnol Biol Med. 2011;7:341–50. doi: 10.1016/j.nano.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Beck-Broichsitter M, Schweiger C, Schmehl T, Gessler T, Seeger W, Kissel T. Characterization of novel spray-dried polymeric particles for controlled pulmonary drug delivery. J Control Release. 2012;158:329–35. doi: 10.1016/j.jconrel.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Bege N, Renette T, Endres T, Beck-Broichsitter M, Hänggi D, Kissel T. In situ forming nimodipine depot system based on microparticles for the treatment of posthemorrhagic cerebral vasospasm. Eur J Pharm Biopharm. 2013;84:99–105. doi: 10.1016/j.ejpb.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Behera T, Swain P. Antigen adsorbed surface modified poly-ɛ-caprolactone microspheres stimulates both adaptive and innate immune response in fish. Vaccine. 2012;30:5278–84. doi: 10.1016/j.vaccine.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Benoit MA, Baras B, Gillard J. Preparation and characterization of protein-loaded poly(epsilon-caprolactone) microparticles for oral vaccine delivery. Int J Pharm. 1999;184:73–84. doi: 10.1016/s0378-5173(99)00109-x. [DOI] [PubMed] [Google Scholar]
- 28.Bernkop-Schnürch A, Heinrich A, Greimel A. Development of a novel method for the preparation of submicron particles based on thiolated chitosan. Eur J Pharm Biopharm. 2006;63:166–72. doi: 10.1016/j.ejpb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Bilati U, Allémann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. doi: 10.1016/j.ejps.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Billon A, Chabaud L, Gouyette A, Bouler J-M, Merle C. Vancomycin biodegradable poly(lactide-co-glycolide) microparticles for bone implantation. influence of the formulation parameters on the size, morphology, drug loading and in vitro release. J Microencapsul. 2005;22:841–52. doi: 10.1080/02652040500162790. [DOI] [PubMed] [Google Scholar]
- 31.Bittner B, Mäder K, Kroll C, Borchert H-H, Kissel T. Tetracycline-hcl-loaded poly(dl-lactide-co-glyco¬lide) microspheres prepared by a spray drying technique: influence of γ-irradiation on radical formation and polymer degradation. J Control Release. 1999;59:23–32. doi: 10.1016/s0168-3659(98)00170-9. [DOI] [PubMed] [Google Scholar]
- 32.Blacklock J, You Y-Z, Zhou Q-H, Mao G, Oupický D. Gene delivery in vitro and in vivo from bioreducible multilayered polyelectrolyte films of plasmid dna. Biomaterials. 2009;30:939–50. doi: 10.1016/j.biomaterials.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozkir A, Saka OM. Formulation and investigation of 5-fu nanoparticles with factorial design-based studies. Il Farm. 2005;60:840–6. doi: 10.1016/j.farmac.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Bruschi M, Cardoso ML, Lucchesi M, Gremião MP. Gelatin microparticles containing propolis obtained by spray-drying technique: preparation and characterization. Int J Pharm. 2003;264:45–55. doi: 10.1016/s0378-5173(03)00386-7. [DOI] [PubMed] [Google Scholar]
- 35.Budhian A, Siegel SJ, Winey KI. Haloperidol-loaded plga nanoparticles: systematic study of particle size and drug content. Int J Pharm. 2007;336:367–75. doi: 10.1016/j.ijpharm.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 36.Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology - towards biomedical applications. Adv Drug Deliv Rev. 2008;60:299–327. doi: 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Cadete A, Figueiredo L, Lopes R, Calado CCR, Almeida AJ, Gonçalves LMD. Development and characterization of a new plasmid delivery system based on chitosan-sodium deoxycholate nanoparticles. Eur J Pharm Sci. 2012;45:451–8. doi: 10.1016/j.ejps.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Cal K, Sollohub K. Spray drying technique. i: hardware and process parameters. J Pharm Sci. 2010;99:575–86. doi: 10.1002/jps.21886. [DOI] [PubMed] [Google Scholar]
- 39.Campos EVR, de Melo NFS, de Paula E, Rosa AH, Fraceto LF. Screening of conditions for the preparation of poly( -caprolactone) nanocapsules containing the local anesthetic articaine. J Colloid Sci Biotechnol. 2013;2:106–11. [Google Scholar]
- 40.Castelli F, Conti B, Maccarrone DE, Conte U, Puglisi G. Comparative study of 'in vitro' release of anti-inflammatory drugs from polylactide-co-glycolide microspheres. Int J Pharm. 1998;176:85–98. [Google Scholar]
- 41.Cevher E, Orhan Z, Mülazımoğlu L, Şensoy D, Alper M, et al. Characterization of biodegradable chitosan microspheres containing vancomycin and treatment of experimental osteomyelitis caused by methicillin-resistant staphylococcus aureus with prepared microspheres. Int J Pharm. 2006;317:127–35. doi: 10.1016/j.ijpharm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS Pharm Sci Tech. 2005;6:E329–57. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chawla JS, Amiji MM. Biodegradable poly(ε-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249:127–38. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 44.Chen A-Z, Zhao Z, Wang S-B, Li Y, Zhao C, Liu Y-G. A continuous ress process to prepare pla–peg–pla microparticles. J Supercrit Fluids. 2011;59:92–7. [Google Scholar]
- 45.Chen Y, Lin X, Park H, Greever R. Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomed Nanotechnol Biol Med. 2009;5:316–22. doi: 10.1016/j.nano.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release. 2002;83:389–400. doi: 10.1016/s0168-3659(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 47.Cırpanlı Y, Allard E, Passirani C, Bilensoy E, Lemaire L, et al. Antitumoral activity of camptothecin-loaded nanoparticles in 9l rat glioma model. Int J Pharm. 2011;403:201–6. doi: 10.1016/j.ijpharm.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Çirpanli Y, Bilensoy E, Lale Doğan A, Çaliş S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camptothecin delivery. Eur J Pharm Biopharm. 2009;73:82–9. doi: 10.1016/j.ejpb.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Cohen-Sela E, Chorny M, Koroukhov N, Danenberg HD, Golomb G. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in plga nanoparticles. J Control Release. 2009;133:90–5. doi: 10.1016/j.jconrel.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 50.Contado C, Vighi E, Dalpiaz A, Leo E. Influence of secondary preparative parameters and aging effects on plga particle size distribution: a sedimentation field flow fractionation investigation. Anal Bioanal Chem. 2013;405:703–11. doi: 10.1007/s00216-012-6113-5. [DOI] [PubMed] [Google Scholar]
- 51.Conti B, Bucolo C, Giannavola C, Puglisi G, Giunchedi P, Conte U. Biodegradable microspheres for the intravitreal administration of acyclovir: in vitro/in vivo evaluation. Eur J Pharm Sci. 1997;5:287–93. [Google Scholar]
- 52.Cruz L, Assumpção E, Andrade SF, Conrado DJ, Kulkamp IC, et al. Gastroresistant microparticles containing sodium alendronate prevent the bone loss in ovariectomized rats. Eur J Pharm Sci. 2010;40:441–7. doi: 10.1016/j.ejps.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Cun D, Foged C, Yang M, Frøkjaer S, Nielsen HM. Preparation and characterization of poly(dl-lactide-co-glycolide) nanoparticles for sirna delivery. Int J Pharm. 2010;390:70–5. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 54.Cun D, Jensen DK, Maltesen MJ, Bunker M, Whiteside P, et al. High loading efficiency and sustained release of sirna encapsulated in plga nanoparticles: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77:26–35. doi: 10.1016/j.ejpb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 55.D’Aurizio E, van Nostrum CF, van Steenbergen MJ, Sozio P, Siepmann F, et al. Preparation and characterization of poly(lactic-co-glycolic acid) microspheres loaded with a labile antiparkinson prodrug. Int J Pharm. 2011;409:289–96. doi: 10.1016/j.ijpharm.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 56.Da Silva AA, Jr, de Matos JR, Formariz TP, Rossanezi G, Scarpa MV, et al. Thermal behavior and stability of biodegradable spray-dried microparticles containing triamcinolone. Int J Pharm. 2009;368:45–55. doi: 10.1016/j.ijpharm.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 57.Dai C, Wang Y, Hou X. Preparation and protein adsorption of porous dextran microspheres. Carbohydr Polym. 2012;87:2338–43. [Google Scholar]
- 58.Dai M, Xu X, Song J, Fu S, Gou M, et al. Preparation of camptothecin-loaded pcec microspheres for the treatment of colorectal peritoneal carcinomatosis and tumor growth in mice. Cancer Lett. 2011;312:189–96. doi: 10.1016/j.canlet.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. Plga-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 60.Das S, Suresh PK, Desmukh R. Design of eudragit rl 100 nanoparticles by nanoprecipitation method for ocular drug delivery. Nanomed Nanotechnol Biol Med. 2010;6:318–23. doi: 10.1016/j.nano.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Dash TK, Konkimalla VB. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: a review. J Control Release. 2012;158:15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 62.De Melo NFS, Campos EVR, de Paula E, Rosa AH, Fraceto LF. Factorial design and characterization studies for articaine hydrochloride loaded alginate/chitosan nanoparticles. J Colloid Sci Biotechnol. 2013;2:146–52. [Google Scholar]
- 63.De Villiers MM, Otto DP, Strydom SJ, Lvov YM. Introduction to nanocoatings produced by layer-by-layer (lbl) self-assembly. Adv Drug Deliv Rev. 2011;63:701–15. doi: 10.1016/j.addr.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Delie F, Berton M, Allémann E, Gurny R. Comparison of two methods of encapsulation of an oligonucleotide into poly(d,l-lactic acid) particles. Int J Pharm. 2001;214:25–30. doi: 10.1016/s0378-5173(00)00627-x. [DOI] [PubMed] [Google Scholar]
- 65.Derakhshandeh K, Fathi S. Role of chitosan nanoparticles in the oral absorption of gemcitabine. Int J Pharm. 2012;437:172–7. doi: 10.1016/j.ijpharm.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Devissaguet J-P, Fessi H, Puisieux F. Process for the preparaton of dispersible colloidal systems of a substance in the form of nanocapsules. US Patent 5049322.1991. [Google Scholar]
- 67.Dhanaraju MD, Jayakumar R, Vamsadhara C. Influence of manufacturing parameters on development of contraceptive steroid loaded injectable microspheres. Chem Pharm Bull (Tokyo) 2004;52:976–9. doi: 10.1248/cpb.52.976. [DOI] [PubMed] [Google Scholar]
- 68.Dinarvand R, Sepehri N, Manoochehri S, Rouhani H, Atyabi F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int J Nanomed. 2011;6:877–95. doi: 10.2147/IJN.S18905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doan TVP, Couet W, Olivier JC. Formulation and in vitro characterization of inhalable rifampicin-loaded plga microspheres for sustained lung delivery. Int J Pharm. 2011;414:112–7. doi: 10.1016/j.ijpharm.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Dong Y, Feng SS. In vitro and in vivo evaluation of methoxy polyethylene glycol–polylactide (mpeg–pla) nanoparticles for small-molecule drug chemotherapy. Biomaterials. 2007;28:4154–60. doi: 10.1016/j.biomaterials.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 71.Dong Y, Feng SS. Methoxy poly(ethylene glycol)-poly(lactide) (mpeg-pla) nanoparticles for controlled delivery of anticancer drugs. Biomaterials. 2004;25:2843–9. doi: 10.1016/j.biomaterials.2003.09.055. [DOI] [PubMed] [Google Scholar]
- 72.Doustgani A, Farahani EV, Imani M, Doulabi AH. Dexamethasone sodium phosphate release from chitosan nanoparticles prepared by ionic gelation method. J Colloid Sci Biotechnol. 2012;1:42–50. [Google Scholar]
- 73.Du W-L, Niu S-S, Xu Y-L, Xu Z-R, Fan C-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym. 2009;75:385–9. [Google Scholar]
- 74.Duchêne D. Cyclodextrins in targeting application to nanoparticles. Adv Drug Deliv Rev. 1999;36:29–40. doi: 10.1016/s0169-409x(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 75.Duchêne D, Ponchel G, Wouessidjewe D. Cyclodextrins in targeting: application to nanoparticles. Adv Drug Deliv Rev. 1999;36:29–40. doi: 10.1016/s0169-409x(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 76.Eidi H, Joubert O, Attik G, Duval RE, Bottin MC, et al. Cytotoxicity assessment of heparin nanoparticles in nr8383 macrophages. Int J Pharm. 2010;396:156–65. doi: 10.1016/j.ijpharm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Elaissari A. Thermally sensitive latex particles. In: Birdi K, editor. Handbook of surface and colloid chemistry. 2nd. Boca Raton, FL: CRC Press; 2002. [Google Scholar]
- 78.Eley JG, Mathew P. Preparation and release characteristics of insulin and insulin-like growth factor-one from polymer nanoparticles. J Microencapsul. 2007;24:225–34. doi: 10.1080/02652040601162335. [DOI] [PubMed] [Google Scholar]
- 79.Elias H-G. An introduction to plastics. Weinheim: Wiley-VCH; 2003. [Google Scholar]
- 80.Elizondo E, Veciana J, Ventosa N. Nanostructuring molecular materials as particles and vesicles for drug delivery, using compressed and supercritical fluids. Nanomedicine. 2012;7:1391–1408. doi: 10.2217/nnm.12.110. [DOI] [PubMed] [Google Scholar]
- 81.Elmizadeh H, Khanmohammadi M, Ghasemi K, Hassanzadeh G, Nassiri-Asl M, Garmarudi AB. Preparation and optimization of chitosan nanoparticles and magnetic chitosan nanoparticles as delivery systems using box–behnken statistical design. J Pharm Biomed Anal. 2013;80:141–6. doi: 10.1016/j.jpba.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 82.Esmaeili A, Saremnia B, Koohian A, Rezazadeh S. Mechanism of nanocapsules of matricaria recutita l. extract formation by the emulsion–diffusion process. Superlattices Microstruct. 2011;50:340–9. [Google Scholar]
- 83.Esposito E, Cervellati F, Menegatti E, Nastruzzi C, Cortesi R. Spray dried eudragit microparticles as encapsulation devices for vitamin C. Int J Pharm. 2002;242:329–34. doi: 10.1016/s0378-5173(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 84.Essa S, Rabanel JM, Hildgen P. Effect of aqueous solubility of grafted moiety on the physicochemical properties of poly(d,l-lactide) (pla) based nanoparticles. Int J Pharm. 2010;388:263–73. doi: 10.1016/j.ijpharm.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 85.Fàbregas A, Miñarro M, García-Montoya E, Pérez-Lozano P, Carrillo C, et al. Impact of physical parameters on particle size and reaction yield when using the ionic gelation method to obtain cationic polymeric chitosan–tripolyphosphate nanoparticles. Int J Pharm. 2013;446:199–204. doi: 10.1016/j.ijpharm.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 86.Falamarzian A, Lavasanifar A. Optimization of the hydrophobic domain in poly(ethylene oxide)-poly(ɛ-caprolactone) based nano-carriers for the solubilization and delivery of amphotericin b. Colloids Surf B Biointerfaces. 2010;81:313–20. doi: 10.1016/j.colsurfb.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 87.Falco N, Reverchon E, Della Porta G. Injectable plga/hydrocortisone formulation produced by continuous supercritical emulsion extraction. Int J Pharm. 2013;441:589–97. doi: 10.1016/j.ijpharm.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 88.Florindo HF, Pandit S, Lacerda L, Gonçalves LMD, Alpar HO, Almeida AJ. The enhancement of the immune response against s. equi antigens through the intranasal administration of poly-ɛ-caprolactone-based nanoparticles. Biomaterials. 2009;30:879–91. doi: 10.1016/j.biomaterials.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 89.Fonseca C, Simões S, Gaspar R. Paclitaxel-loaded plga nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. J Control Release. 2002;83:273–86. doi: 10.1016/s0168-3659(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 90.Fredriksen BN, Grip J. Plga/pla micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of atlantic salmon (salmo salar l.) Vaccine. 2012;30:656–67. doi: 10.1016/j.vaccine.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 91.Gaignaux A, Réeff J, Siepmann F, Siepmann J, De Vriese C, et al. Development and evaluation of sustained-release clonidine-loaded plga microparticles. Int J Pharm. 2012;437:20–8. doi: 10.1016/j.ijpharm.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Galindo-Rodríguez SA, Puel F, Briançon S, Allémann E, Doelker E, Fessi H. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur J Pharm Sci. 2005;25:357–67. doi: 10.1016/j.ejps.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 93.Gan Q, Wang T, Cochrane C, McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan–tpp nanoparticles intended for gene delivery. Colloids Surf B Biointerfaces. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Gander B, Johansen P, Nam-Trân H, Merkle HP. Thermodynamic approach to protein microencapsulation into poly(d,l-lactide) by spray drying. Int J Pharm. 1996;129:51–61. [Google Scholar]
- 95.Garekani HA, Moghaddam ZF, Sadeghi F. Organic solution versus aqueous dispersion of eudragit rs in preparation of sustained release microparticles of theophylline using spray drying. Colloids Surf B Biointerfaces. 2013;108:374–9. doi: 10.1016/j.colsurfb.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 96.Gennes P-G. Scaling concepts in polymer physics. Ithaca, NY: Cornell University Press; 1979. [Google Scholar]
- 97.Gèze A, Aous S, Baussanne I, Putaux J, Defaye J, Wouessidjewe D. Influence of chemical structure of amphiphilic β-cyclodextrins on their ability to form stable nanoparticles. Int J Pharm. 2002;242:301–5. doi: 10.1016/s0378-5173(02)00192-8. [DOI] [PubMed] [Google Scholar]
- 98.Giri TK, Choudhary C, Ajazuddin, Alexander A, Badwaik H, Tripathi DK. Prospects of pharmaceuticals and biopharmaceuticals loaded microparticles prepared by double emulsion technique for controlled delivery. Saudi Pharm J. 2013;21:125–41. doi: 10.1016/j.jsps.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giunchedi P, Juliano C, Gavini E, Cossu M, Sorrenti M. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur J Pharm Biopharm. 2002;53:233–9. doi: 10.1016/s0939-6411(01)00237-5. [DOI] [PubMed] [Google Scholar]
- 100.Gou M, Zheng L, Peng X, Men K, Zheng X, et al. Poly(ɛ-caprolactone)–poly(ethylene glycol)–poly(ɛ-caprolactone) (pcl–peg–pcl) nanoparticles for honokiol delivery in vitro. Int J Pharm. 2009;375:170–6. doi: 10.1016/j.ijpharm.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 101.Govender T, Riley T, Ehtezazi T, Garnett MC, Stolnik S, et al. Defining the drug incorporation properties of pla–peg nanoparticles. Int J Pharm. 2000;199:95–110. doi: 10.1016/s0378-5173(00)00375-6. [DOI] [PubMed] [Google Scholar]
- 102.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. Plga nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Control Release. 1999;57:171–85. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 103.Guenet J-M. Thermoreversible gelation of polymers and biopolymers. New York: Academic Press; 1992. [Google Scholar]
- 104.Guerreiro LH, Da Silva D, Ricci-Junior E, Girard-Dias W, Mascarenhas CM, et al. Polymeric particles for the controlled release of human amylin. Colloids Surf B Biointerfaces. 2012;94:101–6. doi: 10.1016/j.colsurfb.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 105.Gupta AP, Kumar V. New emerging trends in synthetic biodegradable polymers – polylactide: a critique. Eur Polym J. 2007;43:4053–74. [Google Scholar]
- 106.Gupta V, Ahsan F. Influence of pei as a core modifying agent on plga microspheres of pge₁, a pulmonary selective vasodilator. Int J Pharm. 2011;413:51–62. doi: 10.1016/j.ijpharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gyulai G, Pénzes CB, Mohai M, Csempesz F, Kiss É. Influence of surface properties of polymeric nanoparticles on their membrane affinity. Eur Polym J. 2013;49:2495–2503. [Google Scholar]
- 108.Hachicha W, Kodjikian L, Fessi H. Preparation of vancomycin microparticles: importance of preparation parameters. Int J Pharm. 2006;324:176–84. doi: 10.1016/j.ijpharm.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Han S, Li M, Liu X, Gao H, Wu Y. Construction of amphiphilic copolymer nanoparticles based on gelatin as drug carriers for doxorubicin delivery. Colloids Surf B Biointerfaces. 2013;102:833–41. doi: 10.1016/j.colsurfb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 110.Hao S, Wang B, Wang Y, Zhu L, Wang B, Guo T. Preparation of eudragit l 100-55 enteric nanoparticles by a novel emulsion diffusion method. Colloids Surf B Biointerfaces. 2013;108:127–33. doi: 10.1016/j.colsurfb.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 111.Haznedar S, Dortunç B. Preparation and in vitro evaluation of eudragit microspheres containing acetazolamide. Int J Pharm. 2004;269:131–40. doi: 10.1016/j.ijpharm.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 112.He C, Yin L, Tang C, Yin C. Multifunctional polymeric nanoparticles for oral delivery of tnf-α sirna to macrophages. Biomaterials. 2013;34:2843–54. doi: 10.1016/j.biomaterials.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 113.He P, Davis SS, Illum L. Chitosan microspheres prepared by spray drying. Int J Pharm. 1999;187:53–65. doi: 10.1016/s0378-5173(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 114.Hegazy N, Demirel M, Yazan Y. Preparation and in vitro evaluation of pyridostigmine bromide microparticles. Int J Pharm. 2002;242:171–4. doi: 10.1016/s0378-5173(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 115.Hernán Pérez de la Ossa D, Ligresti A, Gil-Alegre ME, Aberturas MR, Molpeceres J, et al. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: development, characterization and in vitro evaluation of their antitumoral efficacy. J Control Release. 2012;161:927–32. doi: 10.1016/j.jconrel.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Hombreiro Pérez M, Zinutti C, Lamprecht A, Ubrich N, Astier A, et al. The preparation and evaluation of poly(ϵ-caprolactone) microparticles containing both a lipophilic and a hydrophilic drug. J Control Release. 2000;65:429–38. doi: 10.1016/s0168-3659(99)00253-9. [DOI] [PubMed] [Google Scholar]
- 117.Hu Y, Jiang X, Ding Y, Zhang L, Yang C, et al. Preparation and drug release behaviors of nimodipine-loaded poly(caprolactone)–poly(ethylene oxide)–polylactide amphiphilic copolymer nanoparticles. Biomaterials. 2003;24:2395–404. doi: 10.1016/s0142-9612(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 118.Huang Y, Gao H, Gou M, Ye H, Liu Y, et al. Acute toxicity and genotoxicity studies on poly(ɛ-caprolactone)-poly(ethylene glycol)-poly(ɛ-caprol¬actone) nanomaterials. Mutat Res Toxicol Environ Mutagen. 2010;696:101–6. doi: 10.1016/j.mrgentox.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 119.Huang Y-Y, Chung T-W, Tzeng T. Drug release from pla/peg microparticulates. Int J Pharm. 1997;156:9–15. [Google Scholar]
- 120.Hyvönen S, Peltonen L, Karjalainen M, Hirvonen J. Effect of nanoprecipitation on the physicochemical properties of low molecular weight poly (l-lactic acid) nanoparticles loaded with salbutamol sulphate and beclomethasone dipropionate. Int J Pharm. 2005;295:269–81. doi: 10.1016/j.ijpharm.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 121.Iler RK. Multilayers of colloidal particles. J Colloid Interface Sci. 1966;21:569–94. [Google Scholar]
- 122.Illum L, Fisher AN, Jabbal-Gill I, Davis SS. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int J Pharm. 2001;222:109–19. doi: 10.1016/s0378-5173(01)00708-6. [DOI] [PubMed] [Google Scholar]
- 123.Ishihara T, Takahashi M, Higaki M, Mizushima Y, Mizushima T. Preparation and characterization of a nanoparticulate formulation composed of peg-pla and pla as anti-inflammatory agents. Int J Pharm. 2010;385:170–5. doi: 10.1016/j.ijpharm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 124.Jafarinejad S, Gilani K, Moazeni E, Ghazi-Khansari M, Najafabadi AR, Mohajel N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012;222:65–70. [Google Scholar]
- 125.Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32:503–15. doi: 10.1016/j.biomaterials.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 126.Javadzadeh Y, Ahadi F, Davaran S, Mohammadi G, Sabzevari A, Adibkia K. Preparation and physicochemical characterization of naproxen-plga nanoparticles. Colloids Surf B Biointerfaces. 2010;81:498–502. doi: 10.1016/j.colsurfb.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 127.Jordan F, Naylor A, Kelly CA, Howdle SM, Lewis A, Illum L. Sustained release hgh microsphere formulation produced by a novel supercritical fluid technology: in vivo studies. J Control Release. 2010;141:153–60. doi: 10.1016/j.jconrel.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 128.Kadam RS, Tyagi P, Edelhauser HF, Kompella UB. Influence of choroidal neovascularization and biodegradable polymeric particle size on transscleral sustained delivery of triamcinolone acetonide. Int J Pharm. 2012;434:140–7. doi: 10.1016/j.ijpharm.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 129.Kalani M, Yunus R. Effect of supercritical fluid density on nanoencapsulated drug particle size using the supercritical antisolvent method. Int J Nanomed. 2012;7:2165–72. doi: 10.2147/IJN.S29805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang Y, Wu J, Yin G, Huang Z, Yao Y, et al. Preparation, characterization and in vitro cytotoxicity of indomethacin-loaded plla/plga microparticles using supercritical co2 technique. Eur J Pharm Biopharm. 2008;70:85–97. doi: 10.1016/j.ejpb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 131.Kang Y, Yang C, Ouyang P, Yin G, Huang Z, et al. The preparation of bsa-plla microparticles in a batch supercritical anti-solvent process. Carbohydr Polym. 2009;77:244–9. [Google Scholar]
- 132.Kanthamneni N, Sharma S, Meenach SA, Billet B, Zhao J-C, et al. Enhanced stability of horseradish peroxidase encapsulated in acetalated dextran microparticles stored outside cold chain conditions. Int J Pharm. 2012;431:101–10. doi: 10.1016/j.ijpharm.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 133.Karataş A, Sonakin O, Kiliçarslan M, Baykara T. Poly (epsilon-caprolactone) microparticles containing levobunolol hcl prepared by a multiple emulsion (w/o/w) solvent evaporation technique: effects of some formulation parameters on microparticle characteristics. J Microencapsul. 2009;26:63–74. doi: 10.1080/02652040802141039. [DOI] [PubMed] [Google Scholar]
- 134.Karnchanajindanun J, Srisa-ard M, Baimark Y. Genipin-cross-linked chitosan microspheres prepared by a water-in-oil emulsion solvent diffusion method for protein delivery. Carbohydr Polym. 2011;85:674–80. [Google Scholar]
- 135.Katara R, Majumdar DK. Eudragit rl 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf B Biointerfaces. 2013;103:455–62. doi: 10.1016/j.colsurfb.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 136.Katare YK, Muthukumaran T, Panda AK. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded pla microparticles. Int J Pharm. 2005;301:149–60. doi: 10.1016/j.ijpharm.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 137.Kauffman KJ, Kanthamneni N, Meenach SA, Pierson BC, Bachelder EM, Ainslie KM. Optimization of rapamycin-loaded acetalated dextran microparticles for immunosuppression. Int J Pharm. 2012;422:356–63. doi: 10.1016/j.ijpharm.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 138.Keegan GM, Smart JD, Ingram MJ, Barnes L-M, Burnett GR, Rees GD. Chitosan microparticles for the controlled delivery of fluoride. J Dent. 2012;40:229–40. doi: 10.1016/j.jdent.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 139.Khaled KA, Sarhan HA, Ibrahim MA, Ali AH, Naguib YW. Prednisolone-loaded plga microspheres. in vitro characterization and in vivo application in adjuvant-induced arthritis in mice. AAPS Pharm Sci Tech. 2010;11:859–69. doi: 10.1208/s12249-010-9445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khalil NM, do Nascimento TCF, Casa DM, Dalmolin LF, de Mattos AC, et al. Pharmacokinetics of curcumin-loaded plga and plga-peg blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–60. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 141.Khalil SKH, El-Feky GS, El-Banna ST, Khalil WA. Preparation and evaluation of warfarin-β-cyclo¬dextrin loaded chitosan nanoparticles for transdermal delivery. Carbohydr Polym. 2012;90:1244–53. doi: 10.1016/j.carbpol.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 142.Khan MS, Vishakante GD, Bathool A. Development and characterization of brimonidine tartrate loaded eudragit nanosuspensions for ocular drug delivery. J Colloid Sci Biotechnol. 2012;1:122–8. doi: 10.1166/jbn.2013.1475. [DOI] [PubMed] [Google Scholar]
- 143.Khayata N, Abdelwahed W, Chehna MF, Charcosset C, Fessi H. Preparation of vitamin e loaded nano-capsules by the nanoprecipitation method: from laboratory scale to large scale using a membrane contactor. Int J Pharm. 2012;423:419–27. doi: 10.1016/j.ijpharm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 144.Khoee S, Sattari A, Atyabi F. Physico-chemical properties investigation of cisplatin loaded polybutyladipate (pba) nanoparticles prepared by w/o/w. Mater Sci Eng C. 2012;32:1078–86. [Google Scholar]
- 145.Khoee S, Yaghoobian M. An investigation into the role of surfactants in controlling particle size of polymeric nanocapsules containing penicillin-g in double emulsion. Eur J Med Chem. 2009;44:2392–9. doi: 10.1016/j.ejmech.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 146.Konecsni K, Low NH, Nickerson MT. Chitosan–tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012;134:1775–9. doi: 10.1016/j.foodchem.2012.03.070. [DOI] [PubMed] [Google Scholar]
- 147.Koten JW, Van Luyn MJA, Cadée JA, Brouwer L, Hennink WE, et al. Il-2 loaded dextran microspheres with attractive histocompatibility properties for local il-2 cancer therapy. Cytokine. 2003;24:57–66. doi: 10.1016/s1043-4666(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 148.Krishnakumar N, Sulfikkarali N, Rajendra Prasad N, Karthikeyan S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (hela) cancer cells. Biomed Prev Nutr. 2011;1:223–31. [Google Scholar]
- 149.Kumar A, Gupta GK, Khedgikar V, Gautam J, Kushwaha P, et al. In vivo efficacy studies of layer-by-layer nano-matrix bearing kaempferol for the conditions of osteoporosis: a study in ovariectomized rat model. Eur J Pharm Biopharm. 2012;82:508–17. doi: 10.1016/j.ejpb.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 150.Kumari A, Yadav SK, Pakade YB, Kumar V, Singh B, et al. Nanoencapsulation and characterization of albizia chinensis isolated antioxidant quercitrin on pla nanoparticles. Colloids Surf B Biointerfaces. 2011;82:224–32. doi: 10.1016/j.colsurfb.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 151.Kwon H-Y, Lee J-Y, Choi S-W, Jang Y, Kim J-H. Preparation of plga nanoparticles containing estrogen by emulsification–diffusion method. Colloids Surf Physicochem Eng Asp. 2001;182:123–30. [Google Scholar]
- 152.Labruère R, Sicard R, Cormier R, Turos E, West L. Poly(vinyl benzoate) nanoparticles for molecular delivery: studies on their preparation and in vitro properties. J Control Release. 2010;148:234–40. doi: 10.1016/j.jconrel.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 153.Lamalle-Bernard D, Munier S, Compagnon C, Charles M-H, Kalyanaraman VS, et al. Coadsorption of hiv-1 p24 and gp120 proteins to surfactant-free anionic pla nanoparticles preserves antigenicity and immunogenicity. J Control Release. 2006;115:57–67. doi: 10.1016/j.jconrel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 154.Le Ray A-M, Chiffoleau S, Iooss P, Grimandi G, Gouyette A, et al. Vancomycin encapsulation in biodegradable poly(ε-caprolactone) microparticles for bone implantation. influence of the formulation process on size, drug loading, in vitro release and cytocompatibility. Biomaterials. 2003;24:443–9. doi: 10.1016/s0142-9612(02)00357-5. [DOI] [PubMed] [Google Scholar]
- 155.Lee SJ, Hong G-Y, Jeong Y-I, Kang M-S, Oh J-S, et al. Paclitaxel-incorporated nanoparticles of hydrophobized polysaccharide and their antitumor activity. Int J Pharm. 2012;433:121–8. doi: 10.1016/j.ijpharm.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 156.Lemos-Senna E, Wouessidjewe D, Duchêne D, Lesieur S. Amphiphilic cyclodextrin nanospheres: particle solubilization and reconstitution by the action of a non-ionic detergent. Colloids Surf B Biointerfaces. 1998;10:291–301. [Google Scholar]
- 157.Leo E, Forni F, Bernabei MT. Surface drug removal from ibuprofen-loaded pla microspheres. Int J Pharm. 2000;196:1–9. doi: 10.1016/s0378-5173(99)00335-x. [DOI] [PubMed] [Google Scholar]
- 158.Li B, Wang Q, Wang X, Wang C, Jiang X. Preparation, drug release and cellular uptake of doxorubicin-loaded dextran-b-poly(ɛ-caprolactone) nanoparticles. Carbohydr Polym. 2013;93:430–7. doi: 10.1016/j.carbpol.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 159.Li P, Wang Y, Peng Z, She F, Kong L. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr Polym. 2011;85:698–704. [Google Scholar]
- 160.Li X, Chang S, Du G, Li Y, Gong J, et al. Encapsulation of azithromycin into polymeric microspheres by reduced pressure-solvent evaporation method. Int J Pharm. 2012;433:79–88. doi: 10.1016/j.ijpharm.2012.04.081. [DOI] [PubMed] [Google Scholar]
- 161.Liang D, Fu X, Liao M, Yuan W, Su J. Development of dextran microparticles loaded with il-1ra of high-encapsulation efficiency and high-bioactivity by a novel method without exposing il-1ra to water–oil interfaces. Powder Technol. 2013;235:299–302. [Google Scholar]
- 162.Limayem Blouza I, Charcosset C, Sfar S, Fessi H. Preparation and characterization of spironolactone-loaded nanocapsules for paediatric use. Int J Pharm. 2006;325:124–31. doi: 10.1016/j.ijpharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 163.Lince F, Marchisio DL, Barresi AA. Strategies to control the particle size distribution of poly-ε-caprolactone nanoparticles for pharmaceutical applications. J Colloid Interface Sci. 2008;322:505–15. doi: 10.1016/j.jcis.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 164.Ma J, Feng P, Ye C, Wang Y, Fan Y. An improved interfacial coacervation technique to fabricate biodegradable nanocapsules of an aqueous peptide solution from polylactide and its block copolymers with poly(ethylene glycol) Colloid Polym Sci. 2001;279:387–92. [Google Scholar]
- 165.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Manchanda R, Fernandez-Fernandez A, Nagesetti A, McGoron AJ. Preparation and characterization of a polymeric (plga) nanoparticulate drug delivery system with simultaneous incorporation of chemotherapeutic and thermo-optical agents. Colloids Surf B Biointerfaces. 2010;75:260–7. doi: 10.1016/j.colsurfb.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 167.Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of dna and sirna. Adv Drug Deliv Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 168.Martin TM, Bandi N, Shulz R, Roberts CB, Kompella UB. Preparation of budesonide and budesonide-pla microparticles using supercritical fluid precipitation technology. AAPS Pharm Sci Tech. 2002;3:E18. doi: 10.1007/BF02830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Martín-Banderas L, Alvarez-Fuentes J, Durán-Lobato M, Prados J, Melguizo C, Fernández-Arévalo M, et al. Cannabinoid derivate-loaded plga nanocarriers for oral administration: formulation, characterization, and cytotoxicity studies. Int J Nanomed. 2012;7:5793–5806. doi: 10.2147/IJN.S34633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Masson V, Maurin F, Fessi H, Devissaguet JP. Influence of sterilization processes on poly(ε-caprolactone) nanospheres. Biomaterials. 1997;18:327–35. doi: 10.1016/s0142-9612(96)00144-5. [DOI] [PubMed] [Google Scholar]
- 171.Maya S, Kumar LG, Sarmento B, Sanoj Rejinold N, Menon D, et al. Cetuximab conjugated o-carboxy-methyl chitosan nanoparticles for targeting egfr overexpressing cancer cells. Carbohydr Polym. 2013;93:661–9. doi: 10.1016/j.carbpol.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 172.Mazzaferro S, Bouchemal K, Maksimenko A, Skanji R, Opolon P, Ponchel G. Reduced intestinal toxicity of docetaxel loaded into mucoadhesive nanoparticles, in mouse xenograft model. J Colloid Sci Biotechnol. 2012;1:210–7. [Google Scholar]
- 173.Mazzarino L, Travelet C, Ortega-Murillo S, Otsuka I, Pignot-Paintrand I, et al. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J Colloid Interface Sci. 2012;370:58–66. doi: 10.1016/j.jcis.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 174.Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release. 2000;69:1–25. doi: 10.1016/s0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- 175.Memişoğlu E, Bochot A, Şen M, Duchêne D, Hıncal AA. Non-surfactant nanospheres of progesterone inclusion complexes with amphiphilic β-cyclo¬dextrins. Int J Pharm. 2003;251:143–53. doi: 10.1016/s0378-5173(02)00593-8. [DOI] [PubMed] [Google Scholar]
- 176.Menei P, Montero-Menei C, Venier M-C, Benoit J-P. Drug delivery into the brain using poly(lactide-co-glycolide) microspheres. Expert Opin Drug Deliv. 2005;2:363–76. doi: 10.1517/17425247.2.2.363. [DOI] [PubMed] [Google Scholar]
- 177.Miladi K, Sfar S, Fessi H, Elaissari A. Drug carriers in osteoporosis: preparation, drug encapsulation and applications. Int J Pharm. 2013;445:181–95. doi: 10.1016/j.ijpharm.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 178.Minost A, Delaveau J, Bolzinger M-A, Fessi H, Elaissari A. Nanoparticles via nanoprecipitation process. Recent Pat Drug Deliv Formul. 2012;6:250–8. doi: 10.2174/187221112802652615. [DOI] [PubMed] [Google Scholar]
- 179.Miyazaki Y, Onuki Y, Yakou S, Takayama K. Effect of temperature-increase rate on drug release characteristics of dextran microspheres prepared by emulsion solvent evaporation process. Int J Pharm. 2006;324:144–51. doi: 10.1016/j.ijpharm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 180.Mo L, Hou L, Guo D, Xiao X, Mao P, Yang X. Preparation and characterization of teniposide plga nanoparticles and their uptake in human glioblastoma u87mg cells. Int J Pharm. 2012;436:815–24. doi: 10.1016/j.ijpharm.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 181.Mohajel N, Najafabadi AR, Azadmanesh K, Vatanara A, Moazeni E, et al. Optimization of a spray drying process to prepare dry powder microparticles containing plasmid nanocomplex. Int J Pharm. 2012;423:577–85. doi: 10.1016/j.ijpharm.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 182.Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–42. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 183.Mora-Huertas CE, Garrigues O, Fessi H, Elaissari A. Nanocapsules prepared via nanoprecipitation and emulsification-diffusion methods: comparative study. Eur J Pharm Biopharm. 2012;80:235–9. doi: 10.1016/j.ejpb.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 184.Morales-Cruz M, Flores-Fernández GM, Morales-Cruz M, Orellano EA, Rodriguez-Martinez JA, et al. Two-step nanoprecipitation for the production of protein-loaded plga nanospheres. Results Pharma Sci. 2012;2:79–85. doi: 10.1016/j.rinphs.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mu L, Feng S. Fabrication, characterization and in vitro release of paclitaxel (taxol®) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release. 2001;76:239–54. doi: 10.1016/s0168-3659(01)00440-0. [DOI] [PubMed] [Google Scholar]
- 186.Muttil P, Kaur J, Kumar K, Yadav AB, Sharma R, Misra A. Inhalable microparticles containing large payload of anti-tuberculosis drugs. Eur J Pharm Sci. 2007;32:140–50. doi: 10.1016/j.ejps.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 187.Nahar M, Mishra D, Dubey V, Jain NK. Development, characterization, and toxicity evaluation of amphotericin b–loaded gelatin nanoparticles. Nanomed Nanotechnol Biol Med. 2008;4:252–61. doi: 10.1016/j.nano.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 188.Nehilla B, Bergkvist M, Popat K, Desai T. Purified and surfactant-free coenzyme q10-loaded biodegradable nanoparticles. Int J Pharm. 2008;348:107–14. doi: 10.1016/j.ijpharm.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 189.O’Donnell PB, McGinity JW. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28:25–42. doi: 10.1016/s0169-409x(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 190.Osman R, Kan PL, Awad G, Mortada N, El-Shamy A-E, Alpar O. Enhanced properties of discrete pulmonary deoxyribonuclease i (dnasei) loaded plga nanoparticles during encapsulation and activity determination. Int J Pharm. 2011;408:257–65. doi: 10.1016/j.ijpharm.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 191.Palazzo F, Giovagnoli S, Schoubben A, Blasi P, Rossi C, Ricci M. Development of a spray-drying method for the formulation of respirable microparticles containing ofloxacin-palladium complex. Int J Pharm. 2013;440:273–82. doi: 10.1016/j.ijpharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 192.Pariot N, Edwards-Lévy F, Andry M-C, Lévy M-C. Cross-linked β-cyclodextrin microcapsules. ii. retarding effect on drug release through semi-permeable membranes. Int J Pharm. 2002;232:175–81. doi: 10.1016/s0378-5173(01)00899-7. [DOI] [PubMed] [Google Scholar]
- 193.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev. 2010;62:28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 194.Patil SB, Sawant KK. Chitosan microspheres as a delivery system for nasal insufflation. Colloids Surf B Biointerfaces. 2011;84:384–9. doi: 10.1016/j.colsurfb.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 195.Péan JM, Venier-Julienne MC, Boury F, Menei P, Denizot B, Benoit JP. Ngf release from poly(d,l-lactide-co-glycolide) microspheres. effect of some formulation parameters on encapsulated ngf stability. J Control Release. 1998;56:175–87. doi: 10.1016/s0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 196.Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Eudragit rs100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16:53–61. doi: 10.1016/s0928-0987(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 197.Pillai O, Panchagnula R. Polymers in drug delivery. Curr Opin Chem Biol. 2001;5:447–51. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 198.Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation i. methods for preparation of drug-loaded polymeric nanoparticles. Nanomed Nanotechnol Biol Med. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 199.Poletto FS, Fiel LA, Donida B, Ré MI, Guterres SS, Pohlmann AR. Controlling the size of poly(hydroxy-butyrate-co-hydroxyvalerate) nanoparticles prepared by emulsification–diffusion technique using ethanol as surface agent. Colloids Surf Physicochem Eng Asp. 2008;324:105–12. [Google Scholar]
- 200.Powell TJ, Palath N, DeRome ME, Tang J, Jacobs A, Boyd JG. Synthetic nanoparticle vaccines produced by layer-by-layer assembly of artificial biofilms induce potent protective t-cell and antibody responses in vivo. Vaccine. 2011;29:558–69. doi: 10.1016/j.vaccine.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 201.Prata AS, Garcia L, Tonon RV, Hubinger MD. Wall material selection for encapsulation by spray drying. J Colloid Sci Biotechnol. 2013;2:86–92. [Google Scholar]
- 202.Prior S, Gamazo C, Irache J, Merkle H, Gander B. Gentamicin encapsulation in pla/plga microspheres in view of treating brucella infections. Int J Pharm. 2000;196:115–25. doi: 10.1016/s0378-5173(99)00448-2. [DOI] [PubMed] [Google Scholar]
- 203.Qi W, Yan X, Fei J, Wang A, Cui Y, Li J. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials. 2009;30:2799–806. doi: 10.1016/j.biomaterials.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 204.Quintanar-Guerrero D, Fessi H, Allémann E, Doelker E. Influence of stabilizing agents and preparative variables on the formation of poly(d,l-lactic acid) nanoparticles by an emulsification-diffusion technique. Int J Pharm. 1996;143:133–41. [Google Scholar]
- 205.Radtchenko IL, Sukhorukov GB, Möhwald H. Incorporation of macromolecules into polyelectrolyte micro- and nanocapsules via surface controlled precipitation on colloidal particles. Colloids Surf Physicochem Eng Asp. 2002;202:127–33. [Google Scholar]
- 206.Raffin RP, Colomé LM, Schapoval EES, Pohlmann AR, Guterres SS. Increasing sodium pantoprazole photostability by microencapsulation: effect of the polymer and the preparation technique. Eur J Pharm Biopharm. 2008;69:1014–8. doi: 10.1016/j.ejpb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 207.Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36:887–913. [Google Scholar]
- 208.Rattes ALR, Oliveira WP. Spray drying conditions and encapsulating composition effects on formation and properties of sodium diclofenac microparticles. Powder Technol. 2007;171:7–14. [Google Scholar]
- 209.Raval JP, Naik DR, Amin KA, Patel PS. Controlled-release and antibacterial studies of doxycycline-loaded poly(ε-caprolactone) microspheres. J Saudi Chem Soc, 2011 Available from: http://dx.doi.org/10.1016/j.jscs.2011.11.004. [Google Scholar]
- 210.Rawat A, Burgess DJ. Effect of ethanol as a processing co-solvent on the plga microsphere characteristics. Int J Pharm. 2010;394:99–105. doi: 10.1016/j.ijpharm.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 211.Rees DA, Welsh EJ. Secondary and tertiary structure of polysaccharides in solutions and gels. Angew Chem Int Ed Engl. 1977;16:214–24. [Google Scholar]
- 212.Rosset V, Ahmed N, Zaanoun I, Stella B, Fessi H, Elaissari A. Elaboration of argan oil nanocapsules containing naproxen for cosmetic and transdermal local application. J Colloid Sci Biotechnol. 2012;1:218–24. [Google Scholar]
- 213.Ruan G, Feng S-S. Preparation and characterization of poly(lactic acid)–poly(ethylene glycol)–poly(lactic acid) (pla–peg–pla) microspheres for controlled release of paclitaxel. Biomaterials. 2003;24:5037–44. doi: 10.1016/s0142-9612(03)00419-8. [DOI] [PubMed] [Google Scholar]
- 214.Sacchetin PSC, Morales AR, Moraes ÂM, e Rosa PTV. Formation of pla particles incorporating 17α-methyltestosterone by supercritical fluid technology. J Supercrit Fluids. 2013;77:52–62. [Google Scholar]
- 215.Sajadi Tabassi SA, Tafaghodi M, Jaafari MR. Induction of high antitoxin titers against tetanus toxoid in rabbits by intranasal immunization with dextran microspheres. Int J Pharm. 2008;360:12–7. doi: 10.1016/j.ijpharm.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 216.Sane A, Limtrakul J. Formation of retinyl palmitate-loaded poly(l-lactide) nanoparticles using rapid expansion of supercritical solutions into liquid solvents (resolv) J Supercrit Fluids. 2009;51:230–7. [Google Scholar]
- 217.Sanli D, Bozbag SE, Erkey C. Synthesis of nanostructured materials using supercritical CO2: Part I. Physical transformations. J Mater Sci. 2012;47:2995–3025. [Google Scholar]
- 218.Santos DT, Albarelli JQ, Beppu MM, Meireles MAA. Stabilization of anthocyanin extract from jabuticaba skins by encapsulation using supercritical co2 as solvent. Food Res Int. 2013;50:617–24. [Google Scholar]
- 219.Sastre RL, Olmo R, Teijón C, Muñíz E, Teijón JM, Blanco MD. 5-fluorouracil plasma levels and biodegradation of subcutaneously injected drug-loaded microspheres prepared by spray-drying poly(d,l-lactide) and poly(d,l-lactide-co-glycolide) polymers. Int J Pharm. 2007;338:180–90. doi: 10.1016/j.ijpharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 220.Seju U, Kumar A, Sawant KK. Development and evaluation of olanzapine-loaded plga nanoparticles for nose-to-brain delivery: in vitro and in vivo studies. Acta Biomater. 2011;7:4169–76. doi: 10.1016/j.actbio.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 221.Seo D-H, Jeong Y-I, Kim D-G, Jang M-J, Jang M-K, Nah J-W. Methotrexate-incorporated polymeric nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan. Colloids Surf B Biointerfaces. 2009;69:157–63. doi: 10.1016/j.colsurfb.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 222.Seremeta KP, Chiappetta DA, Sosnik A. Poly(ε-caprolactone), eudragit® rs 100 and poly(ε-caprolactone)/eudragit® rs 100 blend submicron particles for the sustained release of the antiretroviral efavirenz. Colloids Surf B Biointerfaces. 2013;102:441–9. doi: 10.1016/j.colsurfb.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 223.Shao Y, Zhu B, Li J, Liu X, Tan X, Yang X. Novel chitosan microsphere-templated microcapsules suitable for spontaneous loading of heparin. Mater Sci Eng C. 2009;29:936–41. [Google Scholar]
- 224.Silva AL, Rosalia RA, Sazak A, Carstens MG, Ossendorp F, et al. Optimization of encapsulation of a synthetic long peptide in plga nanoparticles: low-burst release is crucial for efficient cd8(+) t cell activation. Eur J Pharm Biopharm. 2013;83:338–45. doi: 10.1016/j.ejpb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 225.Simşek S, Eroğlu H, Kurum B, Ulubayram K. Brain targeting of atorvastatin loaded amphiphilic plga-b-peg nanoparticles. J Microencapsul. 2013;30:10–20. doi: 10.3109/02652048.2012.692400. [DOI] [PubMed] [Google Scholar]
- 226.Singh M, Kazzaz J, Ugozzoli M, Chesko J, O’Hagan DT. Charged polylactide co-glycolide microparticles as antigen delivery systems. Expert Opin Biol Ther. 2004;4:483–91. doi: 10.1517/14712598.4.4.483. [DOI] [PubMed] [Google Scholar]
- 227.Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-epsilon-caprolactone microspheres and nanospheres: an overview. Int J Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 228.Siqueira-Moura MP, Primo FL, Espreafico EM, Tedesco AC. Development, characterization, and photocytotoxicity assessment on human melanoma of chloroaluminum phthalocyanine nanocapsules. Mater Sci Eng C. 2013;33:1744–52. doi: 10.1016/j.msec.2012.12.088. [DOI] [PubMed] [Google Scholar]
- 229.Soares ASP. A special issue on applications of microencapsulation. J Colloid Sci Biotechnol. 2013;2:77. [Google Scholar]
- 230.Sollohub K, Cal K. Spray drying technique: ii. current applications in pharmaceutical technology. J Pharm Sci. 2010;99:587–97. doi: 10.1002/jps.21963. [DOI] [PubMed] [Google Scholar]
- 231.Song KC, Lee HS, Choung IY, Cho KI, Ahn Y, Choi EJ. The effect of type of organic phase solvents on the particle size of poly(d,l-lactide-co-glycolide) nanoparticles. Colloids Surf Physicochem Eng Asp. 2006;276:162–7. [Google Scholar]
- 232.Song X, Zhao Y, Wu W, Bi Y, Cai Z, et al. Plga nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int J Pharm. 2008;350:320–9. doi: 10.1016/j.ijpharm.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 233.Sou T, Meeusen EN, de Veer M, Morton DAV, Kaminskas LM, McIntosh MP. New developments in dry powder pulmonary vaccine delivery. Trends Biotechnol. 2011;29:191–8. doi: 10.1016/j.tibtech.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 234.Souguir H, Salaün F, Douillet P, Vroman I, Chatterjee S. Nanoencapsulation of curcumin in polyurethane and polyurea shells by an emulsion diffusion method Chem Eng J. 2013;221:133–45. [Google Scholar]
- 235.Stulzer HK, Tagliari MP, Parize AL, Silva MAS, Laranjeira MCM. Evaluation of cross-linked chitosan microparticles containing acyclovir obtained by spray-drying. Mater Sci Eng C. 2009;29:387–92. [Google Scholar]
- 236.Su Z, Sun F, Shi Y, Jiang C, Meng Q, et al. Effects of formulation parameters on encapsulation efficiency and release behavior of risperidone poly(d,l-lactide-co-glycolide) microsphere. Chem Pharm Bull (Tokyo) 2009;57:1251–6. doi: 10.1248/cpb.57.1251. [DOI] [PubMed] [Google Scholar]
- 237.Sukhorukov GB, Donath E, Lichtenfeld H, Knippel E, Knippel M, et al. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf Physicochem Eng Asp. 1998;137:253–66. [Google Scholar]
- 238.Suksiriworapong J, Sripha K, Kreuter J, Junyaprasert VB. Functionalized (poly(ɛ-caprolactone))2-poly(ethyleneglycol) nanoparticles with grafting nicotinic acid as drug carriers. Int J Pharm. 2012;423:562–70. doi: 10.1016/j.ijpharm.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 239.Surassmo S, Min S-G, Bejrapha P, Choi M-J. Effects of surfactants on the physical properties of capsicum oleoresin-loaded nanocapsules formulated through the emulsion–diffusion method. Food Res Int. 2010;43:8–17. [Google Scholar]
- 240.Tawfeek HM. Evaluation of peg and mpeg-co-(pga-co-pdl) microparticles loaded with sodium diclofenac. Saudi Pharm J. 2013;21:387–97. doi: 10.1016/j.jsps.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tewa-Tagne P, Briançon S, Fessi H. Spray-dried microparticles containing polymeric nanocapsules: formulation aspects, liquid phase interactions and particles characteristics. Int J Pharm. 2006;325:63–74. doi: 10.1016/j.ijpharm.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 242.Thomas C, Gupta V, Ahsan F. Influence of surface charge of plga particles of recombinant hepatitis b surface antigen in enhancing systemic and mucosal immune responses. Int J Pharm. 2009;379:41–50. doi: 10.1016/j.ijpharm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 243.Trapani A, Laquintana V, Denora N, Lopedota A, Cutrignelli A, et al. Eudragit rs 100 microparticles containing 2-hydroxypropyl-β-cyclodextrin and glutathione: physicochemical characterization, drug release and transport studies. Eur J Pharm Sci. 2007;30:64–74. doi: 10.1016/j.ejps.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 244.Tse MT, Blatchford C, Oya Alpar H. Evaluation of different buffers on plasmid dna encapsulation into plga microparticles. Int J Pharm. 2009;370:33–40. doi: 10.1016/j.ijpharm.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 245.Valot P, Baba M, Nedelec J-M, Sintes-Zydowicz N. Effects of process parameters on the properties of biocompatible ibuprofen-loaded microcapsules. Int J Pharm. 2009;369:53–63. doi: 10.1016/j.ijpharm.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 246.Van de Ven H, Paulussen C, Feijens PB, Matheeussen A, Rombaut P, et al. Plga nanoparticles and nanosuspensions with amphotericin b: potent in vitro and in vivo alternatives to fungizone and ambisome. J Control Release. 2012;161:795–803. doi: 10.1016/j.jconrel.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 247.Van de Ven H, Vermeersch M, Matheeussen A, Vandervoort J, Weyenberg W, et al. Plga nanoparticles loaded with the antileishmanial saponin β-aescin: factor influence study and in vitro efficacy evaluation. Int J Pharm. 2011;420:122–32. doi: 10.1016/j.ijpharm.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 248.Van der Graaf S, Schroën CGPH, Boom RM. Preparation of double emulsions by membrane emulsification—a review. J Membr Sci. 2005;251:7–15. [Google Scholar]
- 249.Vanderhoff JW, El-Aasser MS, Ugelstad J. Polymer emulsification process. U.S. Patent 4,177,177.Dec, 1979. [Google Scholar]
- 250.Vandervoort J, Ludwig A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur J Pharm Biopharm. 2004;57:251–61. doi: 10.1016/S0939-6411(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 251.Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26:1025–58. doi: 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- 252.Vergaro V, Scarlino F, Bellomo C, Rinaldi R, Vergara D, et al. Drug-loaded polyelectrolyte microcapsules for sustained targeting of cancer cells. Adv Drug Deliv Rev. 2011;63:847–64. doi: 10.1016/j.addr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 253.Vila A, Gill H, McCallion O, Alonso MJ. Transport of pla-peg particles across the nasal mucosa: effect of particle size and peg coating density. J Control Release. 2004;98:231–44. doi: 10.1016/j.jconrel.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 254.Vila A, Sánchez A, Évora C, Soriano I, McCallion O, Alonso MJ. Pla-peg particles as nasal protein carriers: the influence of the particle size. Int J Pharm. 2005;292:43–52. doi: 10.1016/j.ijpharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 255.Vladisavljević GT, Williams RA. Recent developments in manufacturing particulate products from double-emulsion templates using membrane and microfluidic devices. In: Aserin A, editor. Multiple emulsions: technology and applications. New York: Wiley; 2008. pp. 121–164. [Google Scholar]
- 256.Wang F-J, Wang C-H. Sustained release of etanidazole from spray dried microspheres prepared by non-halogenated solvents. J Control Release. 2002;81:263–80. doi: 10.1016/s0168-3659(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 257.Wang G, Yu B, Wu Y, Huang B, Yuan Y, Liu CS. Controlled preparation and antitumor efficacy of vitamin e tpgs-functionalized plga nanoparticles for delivery of paclitaxel. Int J Pharm. 2013;446:24–33. doi: 10.1016/j.ijpharm.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 258.Wang S, Guo S, Cheng L. Disodium norcantharidate loaded poly(ɛ-caprolactone) microspheres: i. preparation and evaluation. Int J Pharm. 2008;350:130–7. doi: 10.1016/j.ijpharm.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 259.Xie H, Smith JW. Fabrication of plga nanoparticles with a fluidic nanoprecipitation system. J Nanobiotechnol. 2010;8:18. doi: 10.1186/1477-3155-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xin H, Chen L, Gu J, Ren X, Wei Z, et al. Enhanced anti-glioblastoma efficacy by ptx-loaded pegylated poly(ɛ-caprolactone) nanoparticles: in vitro and in vivo evaluation. Int J Pharm. 2010;402:238–47. doi: 10.1016/j.ijpharm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 261.Yadav KS, Sawant KK. Formulation optimization of etoposide loaded plga nanoparticles by double factorial design and their evaluation. Curr Drug Deliv. 2010;7:51–64. doi: 10.2174/156720110790396517. [DOI] [PubMed] [Google Scholar]
- 262.Yan S, Zhu J, Wang Z, Yin J, Zheng Y, Chen X. Layer-by-layer assembly of poly(l-glutamic acid)/chitosan microcapsules for high loading and sustained release of 5-fluorouracil. Eur J Pharm Biopharm. 2011;78:336–45. doi: 10.1016/j.ejpb.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 263.Yang J, Park S-B, Yoon H-G, Huh Y-M, Haam S. Preparation of poly ɛ-caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int J Pharm. 2006;324:185–90. doi: 10.1016/j.ijpharm.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 264.Yang Z, Birkenhauer P, Julmy F, Chickering D, Ranieri JP, et al. Sustained release of heparin from polymeric particles for inhibition of human vascular smooth muscle cell proliferation. J Control Release. 1999;60:269–77. doi: 10.1016/s0168-3659(99)00078-4. [DOI] [PubMed] [Google Scholar]
- 265.Yildiz A, John E, Özsoy Y, Araman A, Birchall JC, et al. Inhaled extended-release microparticles of heparin elicit improved pulmonary pharmacodynamics against antigen-mediated airway hyper-reactivity and inflammation. J Control Release. 2012;162:456–63. doi: 10.1016/j.jconrel.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 266.Yoncheva K, Gómez S, Campanero MA, Gamazo C, Irache JM. Bioadhesive properties of pegylated nanoparticles. Expert Opin Drug Deliv. 2005;2:205–18. doi: 10.1517/17425247.2.2.205. [DOI] [PubMed] [Google Scholar]
- 267.Yoo M-K, Kang S-K, Choi J-H, Park I-K, Na H-S, et al. Targeted delivery of chitosan nanoparticles to peyer’s patch using m cell-homing peptide selected by phage display technique. Biomaterials. 2010;31:7738–47. doi: 10.1016/j.biomaterials.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 268.Yordanov G. Influence of the preparation method on the physicochemical properties of econazole-loaded poly(butyl cyanoacrylate) colloidal nanoparticles. Colloids Surf Physicochem Eng Asp. 2012;413:260–5. [Google Scholar]
- 269.Youm I, Murowchick JB, Youan B-BC. Entrapment and release kinetics of furosemide from pegylated nanocarriers. Colloids Surf B Biointerfaces. 2012;94:133–42. doi: 10.1016/j.colsurfb.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 270.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–74. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 271.Zakeri-Milani P, Loveymi BD, Jelvehgari M, Valizadeh H. The characteristics and improved intestinal permeability of vancomycin plga-nanoparticles as colloidal drug delivery system. Colloids Surf B Biointerfaces. 2013;103:174–81. doi: 10.1016/j.colsurfb.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 272.Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E, et al. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J Control Release. 1998;50:31–40. doi: 10.1016/s0168-3659(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 273.Zandanel C, Vauthier C. Poly (isobutylcyanoacrylate) nanoparticles decorated with chitosan: effect of conformation of chitosan chains at the surface on complement activation properties. J Colloid Sci Biotechnol. 2012;1:68–81. [Google Scholar]
- 274.Zhang C, Li G, Wang Y, Cui F, Zhang J, Huang Q. Preparation and characterization of 5-fluorouracil-loaded plla-peg/peg nanoparticles by a novel supercritical CO2 technique. Int J Pharm. 2012;436:272–81. doi: 10.1016/j.ijpharm.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 275.Zhang F, Wu Q, Chen Z-C, Zhang M, Lin X-F. Hepatic-targeting microcapsules construction by self-assembly of bioactive galactose-branched polyelectrolyte for controlled drug release system. J Colloid Interface Sci. 2008;317:477–84. doi: 10.1016/j.jcis.2007.09.065. [DOI] [PubMed] [Google Scholar]
- 276.Zhang F, Wu Q, Liu L-J, Chen Z-C, Lin X-F. Thermal treatment of galactose-branched polyelectrolyte microcapsules to improve drug delivery with reserved targetability. Int J Pharm. 2008;357:22–31. doi: 10.1016/j.ijpharm.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 277.Zhang H, Cui W, Bei J, Wang S. Preparation of poly(lactide-co-glycolide-co-caprolactone) nanoparticles and their degradation behaviour in aqueous solution. Polym Degrad Stab. 2006;91:1929–36. [Google Scholar]
- 278.Zhao H, Gagnon J, Hafeli UO. Process and formulation variables in the preparation of injectable and biodegradable magnetic microspheres. Biomagn Res Technol. 2007;5:2. doi: 10.1186/1477-044X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zheng X, Kan B, Gou M, Fu S, Zhang J, et al. Preparation of mpeg–pla nanoparticle for honokiol delivery in vitro. Int J Pharm. 2010;386:262–7. doi: 10.1016/j.ijpharm.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 280.Zhou YZ, Alany RG, Chuang V, Wen J. Optimization of plga nanoparticles formulation containing l-dopa by applying the central composite design. Drug Dev Ind Pharm. 2013;39:321–30. doi: 10.3109/03639045.2012.681054. [DOI] [PubMed] [Google Scholar]


