Abstract
Objective
To establish prospectively objective selection criteria for metastasectomy in patients with metastatic renal cell carcinoma (mRCC), as selection criteria for metastasectomy in are not well established.
Patients and methods
Between 1991 and 1999, 38 patients with mRCC with responsive or stable disease after initial systemic therapy, and with potentially resectable disease, were enrolled. Patients had a metastasectomy with curative intent and received consolidative adjuvant systemic therapy.
Results
Of the patients enrolled, 79% had stable disease after initial systemic therapy and 21% had a partial or complete response. Most (84%) had metastasectomy of one organ site. There was surgically no evidence of disease (sNED) in 76%. Operative morbidity and mortality were acceptable and 90% of the patients received adjuvant systemic therapy. The median (95% confidence interval) survival was 4.7 (3.0–7.8) years, and the median time to progression was 1.8 (0.8–3.1) years. Eight of 38 patients (21%) remained free of disease by the end of the study. Significant predictors of outcome were lack of sNED after metastasectomy, and the presence of pulmonary metastases. The median overall survival for those who had sNED was 5.6 years, vs 1.4 years for those who did not (P < 0.001).
Conclusions
Metastasectomy in patients with mRCC not progressing after systemic therapy is feasible, with acceptable morbidity. Predictive factors for long-term outcome include pulmonary metastases and sNED. Future work evaluating treatments that can convert many patients into surgical candidates will increase the cure rate of patients with mRCC.
Keywords: renal cell carcinoma, metastasectomy, metastatic
Introduction
RCC accounts for > 90% of kidney cancer cases and ≈ 3% of adult malignancies; > 40 000 individuals will be diagnosed with RCC in the USA in 2008 [1]. Nearly a third of patients with RCC present with metastatic disease (mRCC) at the time of diagnosis and about half develop a recurrence after nephrectomy for localized disease.
Long-term disease-free survival occurs in a small minority of patients with mRCC. The reported 5-year survival remains very low, and ≈ 13 000 patients died from the disease in the USA in 2007 [1]. RCC is resistant to most chemotherapeutic agents and only 10–25% of patients have an objective response to interleukin-2 (IL-2)- or interferon-α-based immunotherapy [2, 3]. Objective responses have also been reported with the use of fluoropyrimidines [4], and most recently, with newer targeted agents including bevacizumab, sunitinib, sorafenib and temsirolimus [5–7]. Although some patients seem to have attained long-term stability of their disease with these therapies, results might be confounded by the well-recognized variability in the natural history of mRCC, which is characterized by periods of stability, even in the absence of therapy. Also, virtually no complete responses (CRs) have been achieved with the newer targeted therapies, suggesting that an integration of systemic therapy with staged debulking surgery might be required to achieve a cure.
Although most patients with mRCC will eventually die from their disease, long-term survival after surgical metastasectomy has been documented in selected patients. In the present study we prospectively explored the concept that an integrated treatment strategy of systemic therapy followed by surgical removal of persistent disease after a period of stability will prolong survival.
Patients and methods
To be eligible for the trial, patients with histologically confirmed RCC had to undergo ≥ 4 months initial treatment with biological agents (i.e. IL-2, interferon-α, interferon-γ, TNF, tumour vaccines) with or without chemotherapy (5-fluorouracil, 5-FU, fluoroxidine), and have either stable or responding disease. Patients with an apparent CR to systemic therapy were excluded. Patients gave informed consent after systemic therapy and before surgery. All patients had radiographically detectable disease before surgery, with a Zubrod performance status of 0–1. To be eligible for systemic therapy after surgery, patients had to have adequate renal (serum creatinine < 2.0 mg/dL), hepatic (bilirubin ≤ 1.5 mg/dL and alanine transferase of ≤ twice the upper limit of normal) and haematological function (absolute neutrophil count > 1500/µL and platelets > 150 000/µL). Patients with serious medical comorbidities were excluded.
After surgery, medically fit patients (with pathologically viable tumour in the surgical specimen) received adjuvant systemic treatment, either the same therapy as before surgery or a combination of interferon-α with 5-FU in a 28-day cycle. Interferon-α was administered subcutaneously daily for 28 days at 5 × 106 U/m2, and 5-FU as a continuous infusion from days 1–5 at a dose of 750 mg/m2 on a 28-day cycle.
Patients were evaluated for the response to systemic therapy after every two cycles of treatment. The WHO criteria were used to define the response, i.e. a CR, disappearance of all radiographic evidence of tumour; partial response (PR), a ≥ 50% decrease in the sum of the products of the diameters of all measured lesions, with no increase in size of lesions or appearance of new lesions. Lytic or mixed bone lesions had to show improvement on bone scans or had to show re-ossification on X-ray films to be considered a partial response; progression of disease, a ≥ 25% increase in the sum of the products of the diameters of any measured lesion or the appearance of new lesion(s). All other responses were defined as stable disease.
Progression-free survival (PFS) was defined as the interval from enrolment in the protocol (after assessment of response to systemic therapy) to disease progression or death. Overall survival (OS) was defined as the interval from protocol enrolment to death. All PFS and OS times were administratively censored as of March 2008. Operative mortality was defined as patients who died within 30 days after metastasectomy and/or during the same hospitalization.
Operability and resectability were determined in a multidisciplinary fashion at the authors’ institution, including a medical oncologist, urologist and radiologist. To define the preoperative extent of disease we used CT of the chest, abdomen, pelvis and brain (if necessary), a chest X-ray and bone scan (bone X-rays and/or CT).
Dedicated genitourinary pathologists evaluated all surgical specimens. No evidence of disease on pathology (pNED) was defined as no macroscopic tumour left behind at the time of the surgery and tumour-free resection margins. A ‘solitary’ site was defined as disease limited to one organ. Bilateral lung involvement was considered ‘solitary organ site’ if no other organ was involved with metastatic disease.
The primary aim of the study was to assess the effect of removing residual tumour and additional systemic therapy on the OS and the PFS of patients with mRCC who had disease regression or stabilization after initial systemic therapy. In all, 40 patients were required to estimate the 2-year survival rate with an SEM of < 8%. Secondary aims included the percentage of patients who were surgically NED (sNED), and the percentage of patients capable of receiving adjuvant systemic therapy.
Univariate data were summarized using standard descriptive statistics. Unadjusted PFS and survival probabilities were estimated using the method of Kaplan and Meier. The Cox proportional hazards regression model was used to assess the ability of patient characteristics to predict survival, with goodness-of-fit assessed by the Grambsch-Therneau test, Martingale residual plots, and likelihood ratio statistics. Predictive variables in the Cox model were selected by performing a backward elimination with P < 0.05 as the threshold and then allowing any variable previously deleted to re-enter the final model once P < 0.05. For statistical computations we used Splus (TIBCO Software Inc, Palo Alto, CA, USA), using both standard Splus functions and the Splus survival analysis package of Therneau.
Results
In all, 38 patients were registered over a 9-year period (1991–99); their characteristics are shown in Table 1. There were 31 men and seven women, with a median (range) age of 55 (36–71) years, typical of patients with mRCC. Almost all (37) patients had a nephrectomy (the one patient who did not had angio-infarction of the affected kidney). The pathology of the primary tumour was conventional RCC in 33 (87%) patients and mixed in five (13%). Four of these five patients had sarcomatoid RCC mixed with clear cell carcinoma, and one had mixed papillary and clear cell carcinoma. Of note, 17 of the 38 patients (45%) had metastatic disease at the initial diagnosis. At the time of first metastasis, 30 patients had single-organ, seven had two-organ and one had three-organ involvement. The most common site of metastasis was the lung. At first diagnosis of metastatic disease, 21 patients had lung metastases, and in 18 the lung was the only site of disease. There was lymph node involvement in 12 patients, five of whom did not have any other site involved, there was renal bed recurrence in six (four as a solitary site), bone metastases in four (three as a solitary site) while other organs (i.e. adrenal gland, pancreas, colon) were involved in four additional patients.
Table 1.
The patients’ characteristics
| Characteristic | N (%) patients or median (range) |
|---|---|
| Total enrolled | 38 |
| Sex (M/F) | 31(82)/7(18) |
| Age, years | 55 (36–71) |
| Interval, months: | |
| first diagnosis to metastasis | 5 (0–264) |
| metastatic disease to metastasectomy | 9 (5–56) |
| Haemoglobin, g/dL, at registration | 12.3 (8.8–15.2) |
| Calcium, g/dL, at registration | 9.5 (1.4–10.8) |
| Systemic therapy before surgery, months | 5 (3–15) |
| Pathology at diagnosis | |
| Conventional | 33 (87) |
| Mixed | 5 (13) |
| Sites of first metastasis | |
| Lung/lung only | 21(55)/18(47) |
| Lymph node/lymph node only | 12(32)/5 (13) |
| Renal bed/renal bed only | 6(16)/4 (10.5) |
| Bone/bone only | 4 (10.5)/3 (8) |
| Other | 4 (10.5) |
| Previous nephrectomy/embolization | 37 (97)/1 (3) |
| Type of previous immunotherapy for metastatic disease | |
| IL-2-containing regimen | 17 (45) |
| Non-IL2-containing regimen | 21 (55) |
| Response to previous immunotherapy | |
| CR or PR | 8 (21) |
| Stable response | 30 (79) |
The median (range) duration of initial systemic treatment was 5 (3–15) months. Seventeen (45%) patients received IL-2 (i.v. or s.c.) as part of their initial systemic therapy (most in combination with interferon-α and 5-FU). Eight patients (21%) achieved a CR or PR to the initial treatment and the remaining 30 (79%) had stable disease.
After the maximum response to systemic therapy, in all 43 surgical procedures were performed in 38 patients; three had two separate procedures (bilateral thoracotomies) each. Thirty-three patients had metastasectomy of one organ site (lung 18, lymph node seven, renal bed five, and bone three) and five of two organ sites each (lung and other in one, lung and lymph node in one, lymph node and other in two, lymph node and bone in one).
The mean (range) hospitalization for metastasectomy was 5 (4–28) days. One patient died within 3 months after surgery before receiving adjuvant therapy. Three patients had significant operative complications (sepsis, respiratory failure with prolonged intubation, and acute pancreatitis, respectively) none of which prevented them from receiving additional systemic therapy.
Of the 38 patients 29 (76%) were rendered sNED; pathological specimens from resected metastatic sites revealed a pathological CR in five cases (13%; bone in two; lung in one, lymph node in one and adrenal gland in one).
After surgery, 34 of 38 patients (89%) received adjuvant systemic treatment, either the same therapy as before surgery or the combination of interferon-α/5-FU therapy described above. Four patients did not receive immunotherapy; one patient died 3 months after surgery, after a cardiac arrest, two progressed within 6 weeks of surgery and one refused to receive adjuvant treatment. Most patients (28/34) received interferon-α + 5-FU as adjuvant therapy for a median of four monthly cycles while six received an IL-2-containing treatment after surgery. Adjuvant systemic treatment was well tolerated. Most patients (29/34) received the planned four monthly cycles. Grade 3 or 4 toxicities were uncommon; of the 34 patients who received adjuvant therapy, five developed Grade 3/4 neutropenia (including two episodes of neutropenic fever), three developed Grade 3/4 stomatitis and one developed Grade 4 diarrhoea.
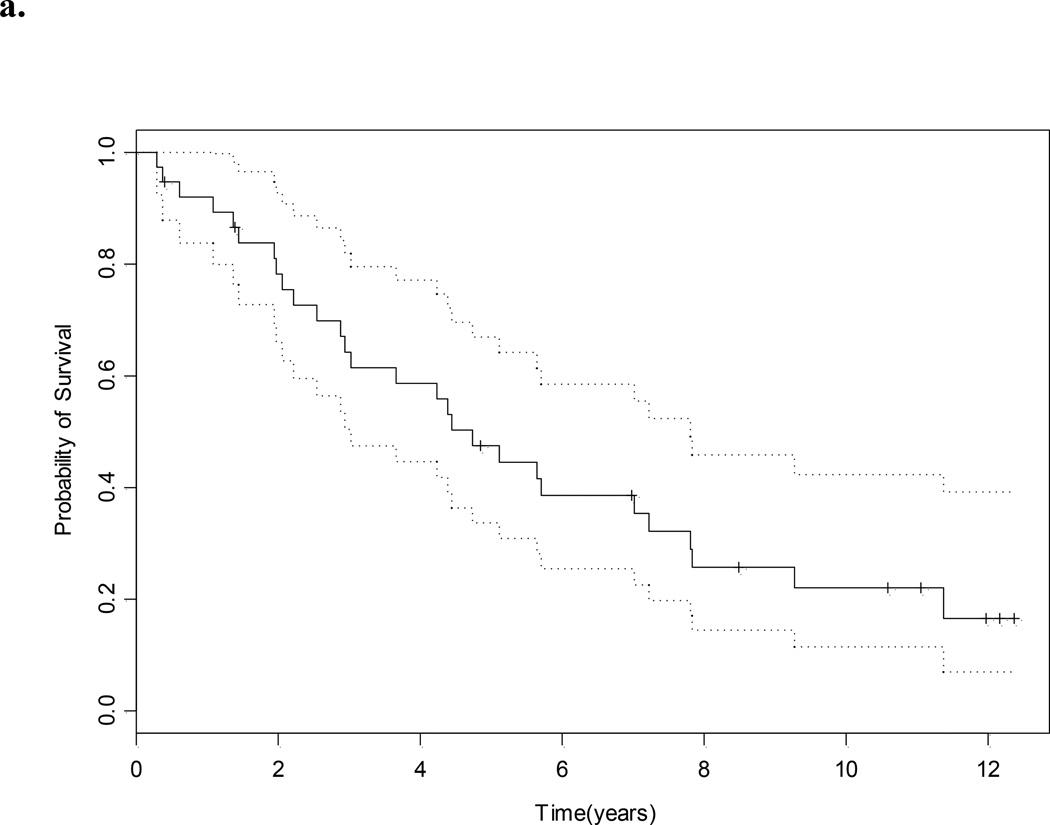
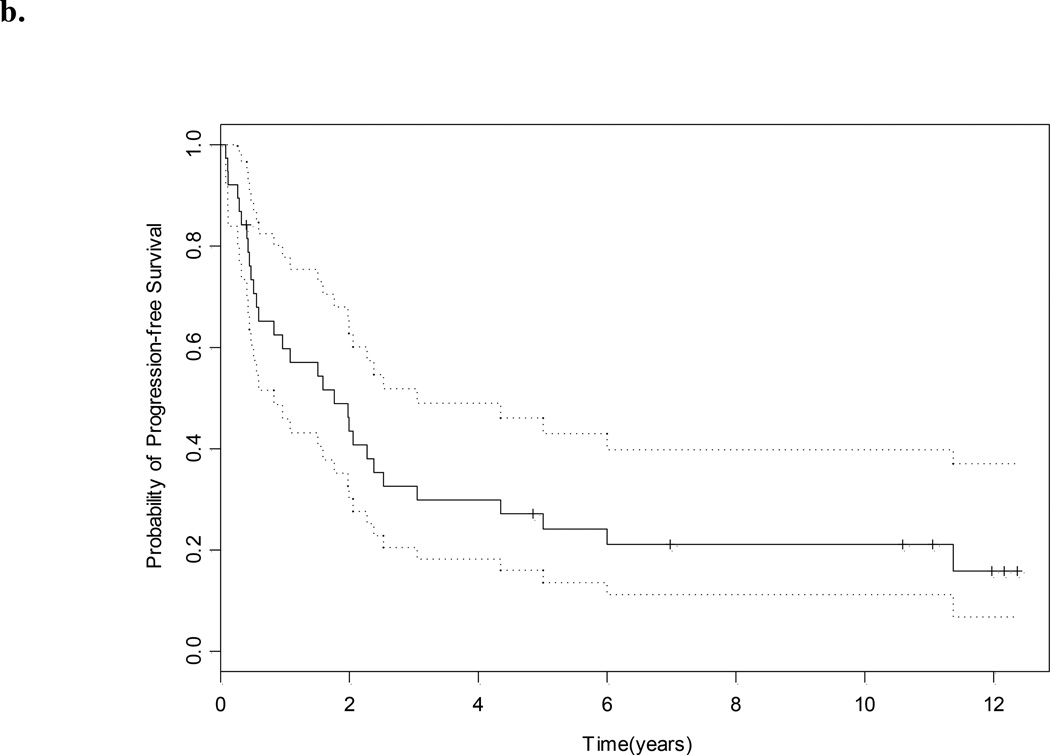
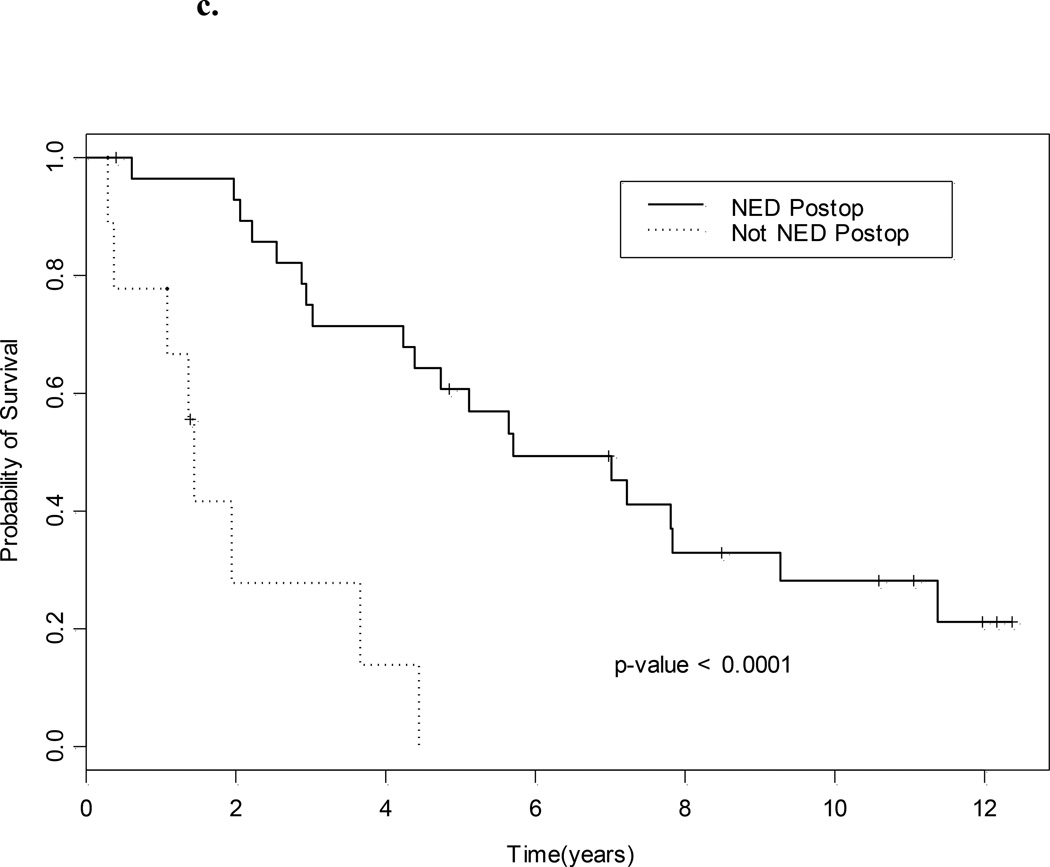
With a median (95% CI) follow-up of 11.1 (10.6 to ?? what is NA –not reached) years the median survival for the whole group was 4.7 (3.0–7.8) years (Fig. 1a). The median PFS was 1.8 (0.8–3.1) years (Fig. 1b), and eight of 38 patients (21%) remain free of disease. The site of relapse in the remaining 30 patients was lung in 14, lymph node in six, bone in nine, adrenal in one, contralateral kidney in two, renal bed in one, soft tissue in two, brain in one, ovary in one and pancreas in one (Table 2). The median OS for those who were sNED was 5.6 years, vs 1.4 years for those who were not (P < 0.001, Fig. 1c).
Fig. 1.
a, OS; b, PFS (in both, dotted lines represent 95% CI); and c, OS and NED status after salvage surgery.
Table 2.
The outcomes of surgery
| Variable | n (%) or median (95% CI) |
|---|---|
| Site of surgery | |
| Lung | 20 (53) |
| Lymph node | 11 (29) |
| Renal bed | 5 (13) |
| Bone | 4 (10.5) |
| Other | 3 (7.9) |
| > 1 site | 5 (13) |
| NED after surgery | 29 (76) |
| Not NED | 9 (24) |
| Type of immunotherapy after surgery | |
| IL-2-containing | 6 (16) |
| Non-IL2-containing | 28 (74) |
| None | 4 (10) |
| PFS, years | 1.8 (0.8–3.1) |
| OS, years | 4.7 (3.0–7.8) |
| OS if pNED | 5.6 |
| OS if not pNED | 1.8 |
Univariate Cox models were fitted for several covariates (Table 3), including previously identified prognostic factors such as calcium, performance status, number of organs involved, lung vs non-lung metastases and time from diagnosis of RCC to metastasis. For OS (Table 3) the only variable that was statistically significant was failure to have sNED after salvage surgery. Univariate analysis for PFS indicated that the presence of pulmonary metastases was associated with an improved outcome, and absence of sNED was associated with worse outcome (Table 3). In a Cox multivariate analysis, lung metastases and absence of sNED were both statistically significant (Table 3), with lung metastases associated with improved outcomes, and absence of sNED associated with worse outcome.
Table 3.
Univariate and multivariate Cox proportional hazards models for OS and PFS
| OS |
PFS |
|||
|---|---|---|---|---|
| Variable | Relative risk | P | Relative risk | P |
| Univariate | ||||
| Age | 1.00 | 0.84 | 1.01 | 0.81 |
| Haemoglobin | 1.13 | 0.41 | 1.15 | 0.33 |
| Calcium | 1.04 | 0.84 | 0.76 | 0.23 |
| Performance status = 1 | 0.63 | 0.29 | 0.74 | 0.47 |
| Pathology = mixed | 0.75 | 0.54 | 0.58 | 0.20 |
| No. organs involved ≥ 2 | 0.92 | 0.86 | 0.85 | 0.74 |
| Metastasis in lung | 0.51 | 0.08 | 0.46 | 0.04 |
| Metastasis in lymph node | 2.00 | 0.09 | 1.67 | 0.20 |
| Metastasis in renal bed | 1.33 | 0.54 | 1.84 | 0.19 |
| Metastasis in bone | 1.08 | 0.90 | 1.28 | 0.69 |
| Metastasis in other | 0.77 | 0.73 | 0.74 | 0.69 |
| Therapy before surgery = No IL-2 | 0.64 | 0.25 | 0.66 | 0.26 |
| Therapy duration, months | 0.97 | 0.62 | 1.01 | 0.90 |
| Log (time first diagnosis to mets) | 1.17 | 0.19 | 1.13 | 0.24 |
| Log (time mets to surgery) | 1.36 | 0.48 | 1.37 | 0.42 |
| No. sites of surgery ≥ 2 | 0.98 | 0.97 | 0.87 | 0.83 |
| Lung at surgery | 0.64 | 0.25 | 0.59 | 0.16 |
| Lymph node at surgery | 1.79 | 0.16 | 1.60 | 0.25 |
| Renal bed at surgery | 1.21 | 0.70 | 1.57 | 0.37 |
| Bone at surgery | 1.08 | 0.90 | 1.28 | 0.69 |
| Other at surgery | 0.47 | 0.47 | 0.39 | 0.36 |
| Not NED after surgery | 7.02 | < 0.001 | 8.71 | < 0.001 |
| Multivariate | ||||
| Not NED after surgery | 6.06 | < 0.001 | 13.98 | < 0.001 |
| Metastasis in lung | 0.62 | 0.003 | 0.30 | 0.003 |
met, metastases.
Discussion
The present study is the first to investigate prospectively the feasibility of curative metastasectomy in patients with mRCC. Several retrospective studies have evaluated this approach. While it is generally believed that operable patients with a solitary lung metastasis derive the greatest benefit from metastasectomy [8, 9], there is significant controversy in other reports about other prognostic factors. For example, a longer time from diagnosis to the development of metastatic disease is a significant prognostic factor in some studies [8–10] but not in others [11, 12]. Similarly, patients with metachronous metastases are reported to have a better outcome in some [10, 13] but not in all [12, 14] studies. A retrospective series from Memorial Sloan-Kettering Cancer Center [8] showed a 5-year OS of 44% for patients who had a ‘curative’ metastasectomy, as opposed to only 14% for those who had non-curative surgery. Multivariate analysis showed that a solitary metastatic site, curative metastasectomy, prolonged time to development of metastatic disease (> 12 months) and male gender were features associated with prolonged survival in that study. By contrast, although solitary metastasis and long disease-free interval were also associated with prolonged survival in a study by Friedel et al. [9], gender, unilateral or bilateral lung involvement or type of surgery were not statistically significant.
The present study showed that failure to have sNED after surgery was the strongest negative predictor of prolonged PFS and OS, and that the presence of lung metastases was a strong predictor of a positive outcome. These observations are in agreement with several previous reports [8, 10, 15, 16]. In addition, metastasectomy of multiple sites did not seem to be associated with worse prognosis than of a solitary metastasis; other studies have shown the same result [11, 14, 17]. Involvement of lymph nodes, renal bed or bone did not favourably or unfavourably influence outcome, suggesting that metastasectomy should not be limited to patients with lung-only disease.
One of the secondary study goals was to determine what percentage of metastases considered resectable by radiographic criteria could be completely resected. Most (76%) of the present patients had a complete metastasectomy. Another goal was to assess what percentage of patients could receive postoperative adjuvant therapy. None of the patients had significant perioperative complications, although one died from sudden cardiac arrest within a month after surgery. Two of 38 patients had an early relapse that precluded further systemic treatment. The vast majority of patients were able to receive 4 months of adjuvant therapy with no significant toxicity. Whether adjuvant systemic therapy provided additional benefit to that obtained from metastasectomy could not be evaluated in this study, because this question was not tested in a randomized fashion.
There are several points about the present data. Although prospective, the study was small. Patients enrolled were carefully selected, and had stable or regressing disease for ≥ 4 months before enrolment. We only included patients with an excellent performance status. In addition, none of these patients received targeted therapy before or after metastasectomy, and as such, these data need to be viewed in the context of the current therapeutic environment.
In conclusion, our prospective study of metastasectomy, combined with the confirmed survival benefit of cytoreductive nephrectomy, support a role for sequential and selective surgery with the intention of removing remaining metastatic disease in the treatment of patients with mRCC. Patients with a good performance status, oligometastatic disease regardless of organ site, and a period of disease stabilization or shrinkage after systemic therapy, appear to be good candidates for surgical removal of their disease. These selection criteria resulted in a high proportion of patients being rendered surgically free of disease, and in a prolonged disease-free and OS. We therefore believe that these criteria serve as a useful guideline for investigators studying the merits of an integrated therapeutic approach. Further prospective studies are needed with newer systemic therapies, and in the context of an improved understanding of how these therapies affect the tumour microenvironment.
Abbreviations
- mRCC
metastatic RCC
- OS
overall survival
- PFS
progression-free survival
- IL-2
interleukin-2
- CR
complete response
- PR
partial response
- 5-FU
5-fluorouracil
- (s)(p)NED
(surgical) (pathological) no evidence of disease
References
- 1.Jemal A, Siegel R, Ward E. Cancer statistics 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Fyfe G, Fisher RI, Rosenberg SA. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 3.Pyrhonen S, Salminen E, Ruutu M. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol. 1999;17:2859–2867. doi: 10.1200/JCO.1999.17.9.2859. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Vogelzang NJ, Dumas MC. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419–2426. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Figlin R, Hutson TE. Sunitinib versus interferon alfa as first-line treatment of metastatic renal cell carcinoma (mRCC): Updated results and analysis of prognostic factors; Presented at the ASCO; 2007. [Google Scholar]
- 7.Hudes G, Carducci M, Tomczak P. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 8.Kavolius JP, Mastorakos DP, Pavlovich C. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261–2266. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 9.Friedel G, Hurtgen M, Penzenstadler M. Resection of pulmonary metastases from renal cell carcinoma. Anticancer Res. 1999;19:1593–1596. [PubMed] [Google Scholar]
- 10.Golimbu M, Joshi P, Sperber A. Renal cell carcinoma. survival and prognostic factors. Urology. 1986;27:291–301. doi: 10.1016/0090-4295(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 11.Kierney PC, van Heerden JA, Segura JW. Surgeon’s role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol. 1994;1:345–352. doi: 10.1007/BF02303572. [DOI] [PubMed] [Google Scholar]
- 12.Wronski M, Arbit E, Russo P. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology. 1996;47:187–193. doi: 10.1016/S0090-4295(99)80413-0. [DOI] [PubMed] [Google Scholar]
- 13.Thrasher JB, Clark JR, Cleland BP. Surgery for pulmonary metastases from renal cell carcinoma. Army experience from 1977 to 1987. Urology. 1990;35:487–491. doi: 10.1016/0090-4295(90)80100-2. [DOI] [PubMed] [Google Scholar]
- 14.Pogrebniak HW, Haas G, Linehan WM. Renal cell carcinoma. resection of solitary and multiple metastases. Ann Thorac Surg. 1992;54:33–38. doi: 10.1016/0003-4975(92)91136-w. [DOI] [PubMed] [Google Scholar]
- 15.van der Poel HG, Roukema JA, Horenblas S. Metastasectomy in renal cell carcinoma: a multicenter retrospective analysis. Eur Urol. 1999;35:197–203. doi: 10.1159/000019849. [DOI] [PubMed] [Google Scholar]
- 16.Piltz S, Meimarakis G, Wichmann MW. Long-term results after pulmonary resection of renal cell carcinoma metastases. Ann Thorac Surg. 2002;73:1082–1087. doi: 10.1016/s0003-4975(01)03602-5. [DOI] [PubMed] [Google Scholar]
- 17.Dernevik L, Berggren H, Larsson S. Surgical removal of pulmonary metastases from renal cell carcinoma. Scand J Urol Nephrol. 1985;19:133–137. doi: 10.3109/00365598509180241. [DOI] [PubMed] [Google Scholar]