Figure 2. Characterization of structural rearrangement of endoplasmic reticulum and evaluation of its calcium capacity after cell loading with magnetic nanoparticles.
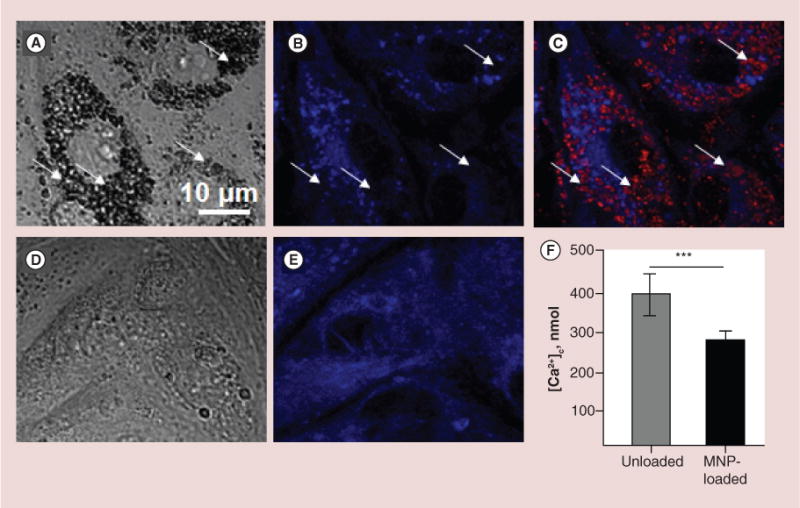
First, cells were loaded for 24 h with BODIPY®564/570-MNPs and after that, ER was visualized using ER-Tracker Blue-White DPX as it is described in the ‘Methods’ section. It was observed that embedding of MNPs into cytosole rearranges ER network, which results in diminished calcium resource of ER. (A–C) Images of primary rat aortic endothelial cells (RAECs) loaded with MNPs. (A) DIC image demonstrates large amount of MNPs in cells seen as black aggregates. (B & C) Confocal images of ER only and merged image of MNPs (red) and ER (blue). Arrows indicate areas where ER was displaced by MNPs. (D & E) DIC and confocal images of MNP-unloaded RAECs. (F) To validate the effect of MPNs on the amount of free calcium in ER, the calcium release was provoked by 1 μM thapsigargin in Ca2+-free buffer containing 1mM EGTA. To avoid leak of calcium released from ER into mitochondria, the mitochondrial calcium uptake was blocked by cell pretreatment with 2 μg/ml oligomycin, which prevents ATP-dependent Ca2+ uniport into mitochondria. Amplitude of cytosolic calcium peak as a result of ER stress was measured fluorimetrically on the confocal microscope. Data presented as mean ± SEM (n = 72 and 75 cells, accordingly), p < 0.0001.
MNP: Magnetic nanoparticle.
