Figure 4. Respiration and oxidative phosphorylation in magnetic nanoparticles-loaded versus unloaded primary rat aortic endothelial cells.
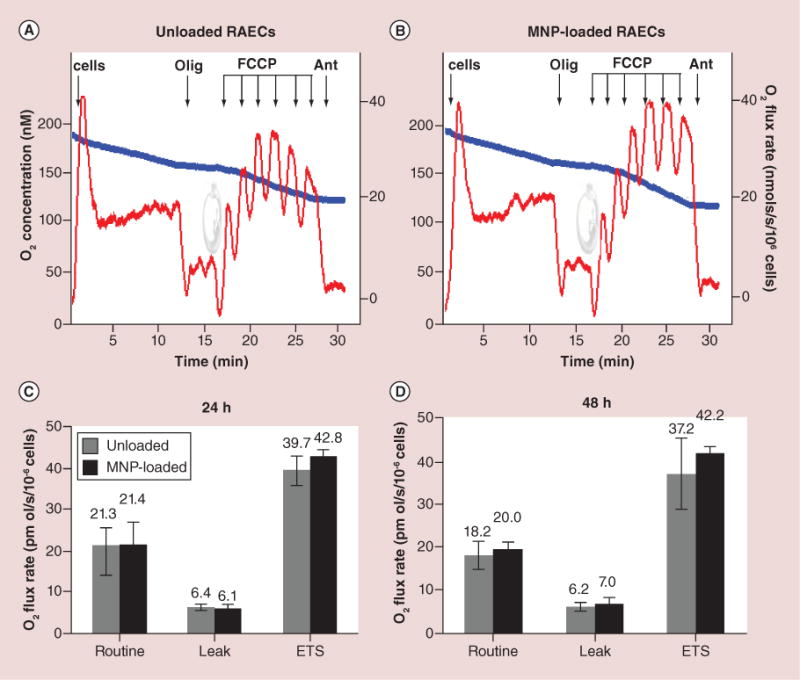
(A & B) The typical steady-state oxygen flux records at different states of uncoupled and stimulated respiration for unloaded and MNP-loaded RAECs. Blue traces are changes in O2 concentration in the closed chamber as a result of oxygen consumption by cellular mitochondria. Red traces are rates of oxygen fluxes calculated using Oroboros DataLab software as the negative time derivative of O2 concentration. (C & D) Respiration data were generated by stepwise analysis of respiratory system constituents at 24 and 48 h after cell loading with MNPs. Routine is a basal nonperturbed respiration. Leak by definition is O2 flux compensating for proton leak measured after inhibition of ATPase with oligomycin. ETS refers to mitochondria maximal respiratory capacity. This maximally stimulated respiration state is induced by titration with low doses of uncoupler FCCP in order to reach the maximally stimulated respiration prior to inhibition of respiration occurs (decline of red traces upon FCCP addition). The addition of antimycin blocks mitochondria respiration. The residual respiration accounting for nonmitochondrial portion of oxygen utilization (residual oxygen consumption) was shown to be similar in both unloaded and loaded cells. Additions: 1 × 106 cell/ml, 2 μg/ml oligomycin, titration by 20 nM dose of FCCP, 2.5 μM antimycin. The numbers above columns are rates of respiration expressed in pmol O2/s/106 cells. Data presented as mean ± SEM (n = 3–4) and are shown to be statistically non-significant.
MNP: Magnetic nanoparticle; RAEC: Primary rat aortic endothelial cell.
