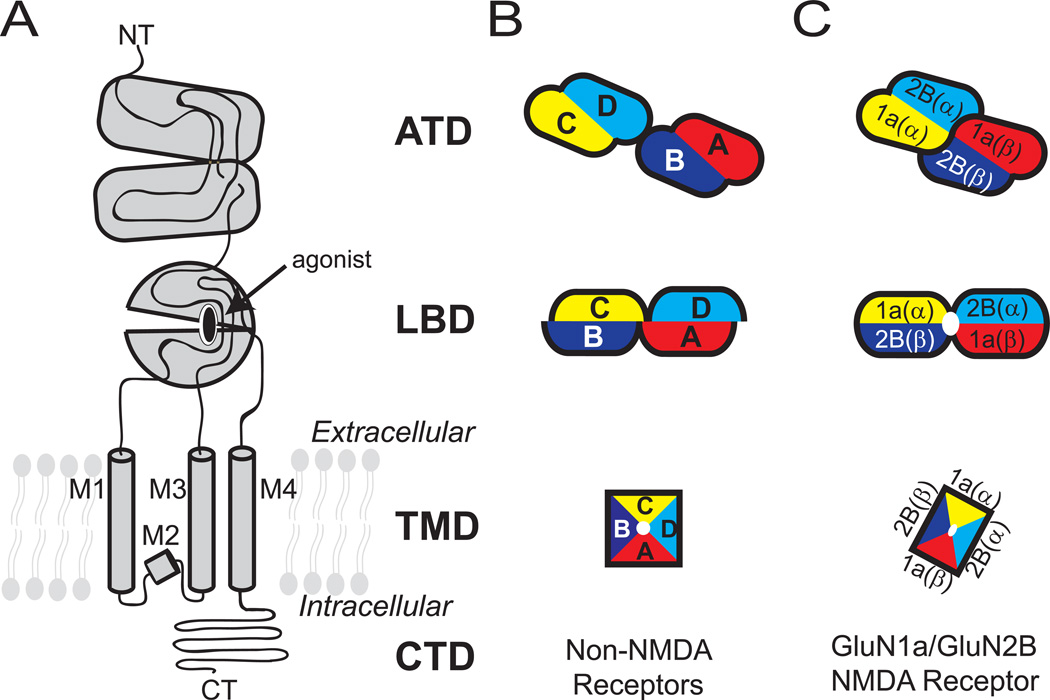
Figure 1. Domain organization and subunit arrangement of iGluRs.
(A) The ionotropic glutamate receptor subunits are composed of distinct domains including the amino terminal domain (ATD), ligand-binding domain (LBD), transmembrane domain (TMD), and carboxyl terminal domain (CTD). The TMD of one subunit is composed of M1-M4 helices. Schematic representation of tetrameric subunit organization of non-NMDARs (B) and NMDARs (C) at ATD, LBD and TMD layers are shown. Dimer pairs at ATD and LBD are indicated by lack of black lines at the interface. The four subunits in non-NMDARs are noted as A-D.