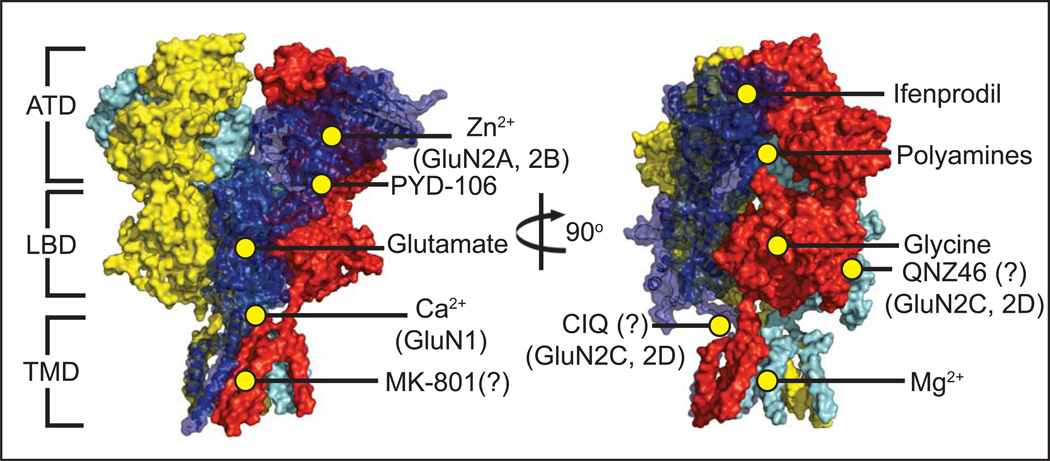
Figure 6. Heterotetrameric GluN1/GluN2B NMDAR and small molecule binding sites.
Numerous ligands have been identified that influence NMDAR activity. Co-agonists glycine [76] and glutamate bind at the LBD of the GluN1 and GluN2 subunits, respectively [77]. The small molecule PYD-106 is a GluN2C-specific allosteric activator which binds at the ATD/LBD interface [70]. Polyamines such as spermine and spermidine bind at the interface of two ATDs in GluN2B-containing receptors and potentiate channel activity [78], but only in GluN1 isoforms lacking Exon5 [79]. A number of NMDAR inhibitors have also been identified, including extracellular Zn2+ [53] binding at the hinge region of the GluN2 ATD [26] (and with a notably stronger effect on GluN2A-containing receptors over other types), and voltage-dependent block by Mg2+ at the TMD [68]. Phenylethanolamines, such as ifenprodil, bind at the interface of the GluN1/GluN2B ATDs [27], while the inhibitor MK-801 is purported to bind very near to the center of the ion channel [54]. A sequence of acidic residues in the LBD/TMD M3 linker in the GluN1 subunit known as the DRPEER motif is essential for calcium binding and conductance [53].