Figure 1. Pyruvate metabolic pathways.
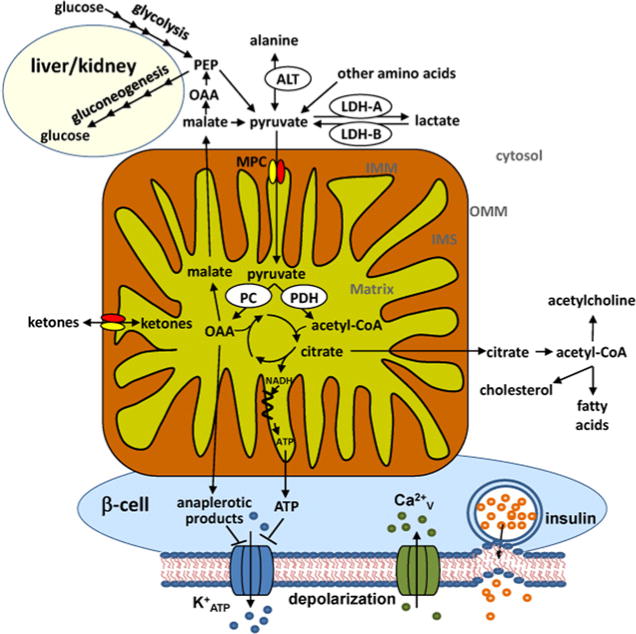
Pyruvate can be formed in the cytosol by glycolysis, or conversion from alanine by ALT, from lactate by LDH-B or from malate by malic enzyme (ME). Pyruvate crosses the outer mitochondrial membrane (OMM) probably via the VDAC into the intermembrane space (IMS). Pyruvate is then transported across the IMM by the MPC. It has also been suggested that the MPC transports ketone bodies across the IMM. In the mitochondrial matrix, pyruvate can be either oxidized into acetyl-CoA by PDH or carboxylated to oxaloacetate (OAA) by PC. Although pyruvate oxidation is important for the production of reducing equivalents for ATP synthesis, citrate formed in the TCA cycle can also be exported to the cytosol, converted to acetyl-CoA, and used to produce new fatty acids, cholesterol or acetylcholine. OAA produced by PC can be exported to the cytosol and converted to phosphoenolpyruvate (PEP), which can then be used to form glucose in gluconeogenic tissues such as the liver, kidney and intestine. Last, both mitochondrial energy produced from pyruvate oxidation and anaplerotic intermediates produced by pyruvate carboxylation play a role in the stimulation of insulin secretion in pancreatic β-cells by inhibiting K+ATP channels, causing depolarization of the plasma membrane, and Ca2+ influx through Ca2+V channels, and allowing insulin secretory vesicle fusion and insulin release.
