Figure 10. Safety and tolerability of the control and macrophage-targeted nanoparticles containing IL-10 plasmid DNA.
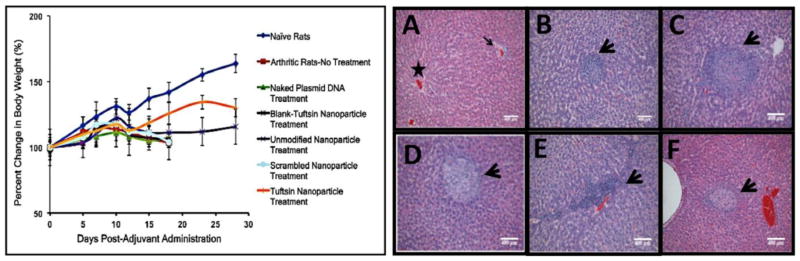
(Left)-Body weight measurements reported as percent change in body weight as a function of day’s post-adjuvant administration. The data is representative as %Mean ± S.D. (n=5 animals/group). Dramatic loss in body weight was observed for arthritis bearing animals (no treatment), and control treatments. Effective therapy with tuftsin-peptide modified nanoparticles encapsulating IL-10 plasmid DNA ameliorated signs of arthritis and accordingly, the treated rats survived for an extended period. Drastic drop in weight after day 23 was attributed to exhaustion of the transient therapeutic effect of IL-10 gene expression. (Right)-H&E staining of the liver to determine alginate based nanoparticles particle-mediated toxicity in arthritis-induced male Lewis rats. The liver samples were collected on day 18, post-adjuvant administration for all the treatment groups. Panel (A) represents Naïve Rats with normal hepatocellular architecture illustrating a central vein (star) and a portal area (arrow). (B)-Arthritis-No Treatment, (C)-Blank-Tuft NP Treatment, (D)-Unmodified Nanoparticle Treatment, (E)-Scrambled-Peptide modified Nanoparticle Treatment, (F) Tuftsin-Peptide modified Nanoparticle Treatment. (n=3 rats/group). Arrow heads in panels A-F represent fibro-histocytic nodules comprising of lymphocytes and plasma cells. These are considered to be common findings observed in the liver of arthritis-bearing rats and are not attributed to nanoparticle administration. Scale Bar = 400μm.
