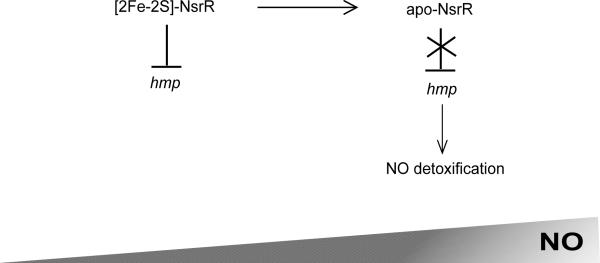
Figure 3. The mechanism of S. Typhimurium NsrR-dependent hmp regulation.
A model of NsrR control of hmp encoding a NO detoxifying flavohaemoglobin under increasing nitric oxide stress. During growth of S. Typhimurium when concentrations of nitric oxide are minimal, such as in the environment outside of the mammalian host, the NsrR [2Fe-2S] cluster is stable. Under these conditions, [2Fe-2S]-NsrR is a functional DNA-binding protein and represses transcription of hmp encoding a nitric oxide detoxifying flavohaemoglobin. However, when S. Typhimurium is exposed to NO stress, such as that generated by host macrophages where S. Typhimurium survives and proliferates during mammalian infection, the NsrR [2Fe-2S] cluster is nitrosylated. These conditions lead to cluster destabilization and abolish NsrR DNA-binding activity. As such, hmp expression is no longer repressed and the resulting NO detoxification by flavohaemoglobin allows for S. Typhimurium persistence within host macrophages.