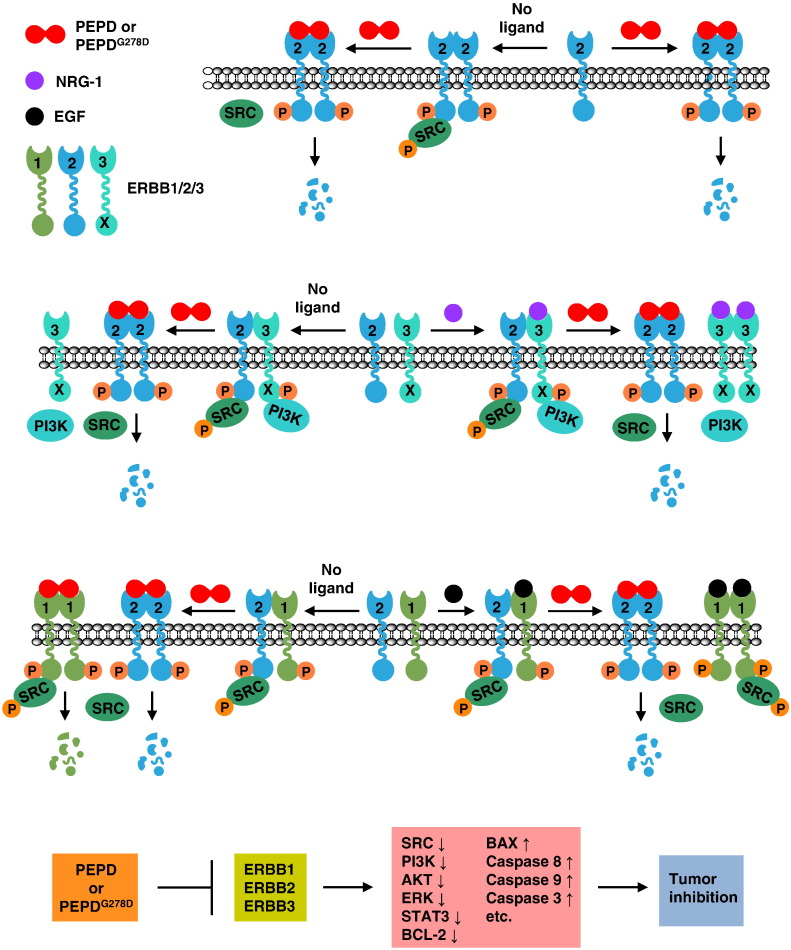
Fig. 6.
Paradigm of molecular changes induced by rhPEPD and rhPEPDG278D in cancer cells. rhPEPD and rhPEPDG278D share the same antitumor mechanism, except that the dipeptidase function of rhPEPD stimulates HIF-1α and other prosurvival factors, which are not shown in the paradigm. The paradigm is constructed based on the present data and those published recently (Yang et al., 2013, Yang et al., 2014). SRC and PI3K are shown as representative downstream signaling molecules. rhPEPD and rhPEPDG278D exist as homodimers and cross-link preformed ERBB2 homodimers, silencing ERBB2-SRC signaling by causing SRC dissociation from ERBB2. They also cross-link ERBB2 monomers, causing dimerization and transient phosphorylation. Upon binding to the PEPDs, ERBB2 is internalized and degraded, leading to profound and persistent ERBB2 depletion. The PEPDs do not bind to ERBB3 but disrupt ligand-independent and NRG-1-induced ERBB2–ERBB3 dimerization and signaling (inactivation of both SRC and PI3K). The PEPDs also cross-link ERBB1, causing dimerization and transient phosphorylation, followed by ERBB1 degradation. Despite binding to both ERBB1 and ERBB2, the PEPDs do not cross-link ERBB1 and ERBB2; rather, they disrupt ligand-independent and EGF-induced ERBB2–ERBB1 dimerization. The PEPDs promote SRC association with ERBB1 homodimer and ERBB1-mediated SRC phosphorylation, but such effect is transient as both agents subsequently cause ERBB1 depletion. Overall, the impact of the PEPDs on ERBB2 and its family members in cancer cells is inhibitory, leading to shutdown of ERBB signaling, induction of apoptosis and inhibition of cancer growth.