Introduction
The biologic response to the presence of a biomaterial, whether metal, ceramic or polymeric in type, is of critical importance to the long-term function of a biomedical implant. It has become clear that although biomaterials may be biocompatible, they are not bio-inert and that a foreign body response is common or universal (Anderson JM et al., 2008), and to some degree biodegradation can be expected including corrosion of metal biomaterials or degradation of polymeric and ceramic materials (Pepas NA and Langer R, 1994).
The most common type of cochlear implant consists of platinum electrodes and a silicone carrier. Histologic evidence of a cellular immune response to the presence of a cochlear implant has been described (Bertuleit GC, et al., 1999; Migirov L, et al., 2001; Kronenberg J et al., 2001; Nadol JB, et al., 2004; Kunda LD, et al., 2006; Ho EC et al., 2007; Nadol JB et al., 2008; Seyyedi M et al., 2014). A foreign body giant cell reaction has been described following implantation of prostheses containing silicone including skin expanders (Maturri L, et al., 1991); arthroplasty prosthetics (Kistler U, et al., 2005); cardiac pacemakers (Maushagen E, et al., 1994); cerebrospinal fluid shunts (Snow R, et al., 1989); and breast implants (Meyer DR, et al., 1998). A cellular tissue response has been described in the presence of platinum in human aneurysm coils (Killer M, et al., 2010) and around platinum eyelid weights (Chan W, et al., 2011).
Histologic evidence of a foreign body reaction has been described in approximately 75 percent (Nadol JB et al., 2008) to 96% (Seyyedi M et al., 2014) of cases in patients who in life had undergone cochlear implantation. In the current study we further characterize the biologic response to cochlear implantation during life using immunohistochemical stains for B cell lymphocytes (CD20), T cell lymphocytes (CD3) and macrophages (CD68). In addition, in order to better characterize the nature of opaque foreign body particles found in proximity to the implant track, energy dispersive x-ray spectroscopy by scanning electron microscopy (EDS-SEM) of adjacent tissue sections was performed. SEM of implanted and unimplanted cochlear implant electrodes was done to test the hypothesis that the opaque foreign body particles seen in tissue macrophages were derived from components of the implanted electrode arrays.
Materials and Methods
Histologic Preparation
Temporal bones were removed after death and fixed in 10% buffered formalin. A CT scan of each specimen was done. The specimens were then decalcified in ethylene/diamine tetra-acetic acid, and the cochlear implant electrode was removed. Processing proceeded with dehydration in graded alcohols, followed by embedment in celloidin. Specimens were then serially sectioned in the axial (horizontal) plane with an average thickness of approximately 20 micrometers. Every tenth section was stained with hematoxylin and eosin and mounted on glass slides for histologic examination and 2-dimensional graphic reconstruction.
Immunohistochemistry Methods
The immunohistochemical characterization of B and T lymphocytes and macrophages was done in seven temporal bones from five individuals (Table). The H&E sections were reviewed for evidence of a cellular immune response in the form of lymphocytic infiltration, macrophages and foreign body giant cells and adjacent unstained sections were prepared for immunostaining. The celloidin sections were mounted on gelatin subbed glass slides. Celloidin was removed with changes of a solution of sodium hydroxide and methanol followed by 100% methanol rinses. The tissue was rehydrated from 100% methanol to 70% methanol and then distilled water. The sections were rinsed in phosphate buffered saline (PBS) and incubated in 5% normal horse serum and then incubated in primary antibodies overnight in a humid chamber at room temperature.
Table.
Cochlear Implantation History in 5 Subjects
| Case #/ear | Age at implantation | Age at death | Duration of implant (Yrs.) | Last WRS/age | Type of Implant |
|---|---|---|---|---|---|
|
| |||||
| 1-AD | 58 | 71 | 13 | Sound awareness/68 | Nucleus 22 |
|
| |||||
| 2-AS | 60 | 71 | 11 | CNC 60%/70 | Nucleus 24 |
| 2-AD | 60 | 71 | 11 | CNC 56%/70 | Nucleus 24 |
|
| |||||
| 3-AS | 59 | 76 | 17 | NU6 0%/50 | Nucleus 22 |
|
| |||||
| 4-AS | 64 | 68 | 4 | HINT 0%/66 | Nucleus 24 |
| 4-AD | 47 | 68 | 21 | HINT 0%/66 | Nucleus 22 |
|
| |||||
| 5-AS | 81 | 91 | 10 | NU6 12%/82 | Nucleus 22 |
Immunostaining for T cells was accomplished using an antibody to CD3 (Ventana Medical Systems, Inc., Tucson, AZ) at a dilution of 1:1 or undiluted. For B cell immunostaining an antibody to CD20 (Ventana Medical Systems, Inc., Tucson, AZ) was used undiluted.
For macrophage immunostaining, an antibody to CD68 (DAKO, Denmark) was used at a dilution of 1:10 or 1:25.
After primary incubation, sections were rinsed in 3 washes of PBS. Secondary antibodies diluted 1:200 and appropriate for the host species of primaries were applied for one hour, and sections were then rinsed 3 times with PBS. Avidin-Biotin-horseradish peroxidase (standard ABC kit, Vector Labs, Burlingame, CA) was applied for one hour and rinsed in PBS. Colorization was accomplished with 0.01% diaminobenzidine and 0.01% hydrogen peroxide (H202) for 5 to 10 minutes and the sections were then rinsed in PBS, dehydrated in graded alcohols and cover slips were applied (O’Malley JT, et al., 2009; O’Malley JT, et al., 2009).
Scanning Electron Microscopy (SEM)
The electrode array was removed after decalcification was complete. The array was then fixed in ½ strength Karnovsky fixative (2% paraformaldehyde plus 2.5% glutaraldehyde in phosphate buffer at a pH 7.4). After fixation, the electrode was rinsed three times in phosphate buffered solution, dehydrated in a graded series of ethanol, critical point dried in a Tousimis Semi-automatic Samdri-795 critical point dryer, and finally coated with a 150 angstrom chromium layer in a Gatan 610 ion beam coater.
The control electrode array which had not been implanted was not fixed but was coated in the same way. Both electrodes were mounted on 12 mm diameter aluminum mounting stubs before the coating was applied. They were then examined in a JEOL 7401F field emission scanning electron microscope.
Energy Dispersive Spectroscopy by Scanning Electron Microscopy (EDS-SEM)
Tissue sections adjacent to those used for immunostaining were prepared in the same way as for immunostaining on glass slides and air dried.
The samples were then examined with a JEOL (Japan Electron Optics Laboratory) model 6610LV scanning electron microscope (SEM) in low vacuum mode with a pressure around 35 Pascals, and images were generated using the Backscatter Electron Detector. Backscatter imaging provides composition information wherein higher atomic number materials appear brighter in the image. These images were useful as a guide as the investigation focused on platinum which has a much higher atomic number (78) than the surrounding organic material. The SEM was equipped with an IXRF energy dispersive spectroscopy system (EDS) which provides elemental identification based on x-ray radiation caused by the electron beam/sample interaction. The IXRF system was equipped with a silicon drift detector, model 550i pulse processor, and Iridium analysis software.
Results
Lymphocytic immunostaining
An infiltration of B lymphocytes (CD20) was frequently seen in proximity to the electrode track as part of the fibrous sheath around the electrode track (Fig. 1A & B). An infiltration of B-lymphocytes was commonly co-populated by T-cells (CD3) (Fig. 2).
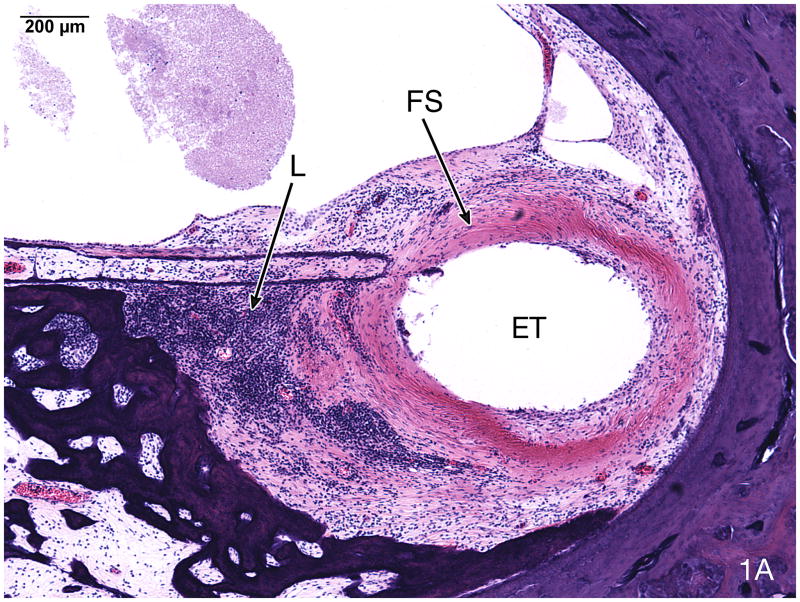
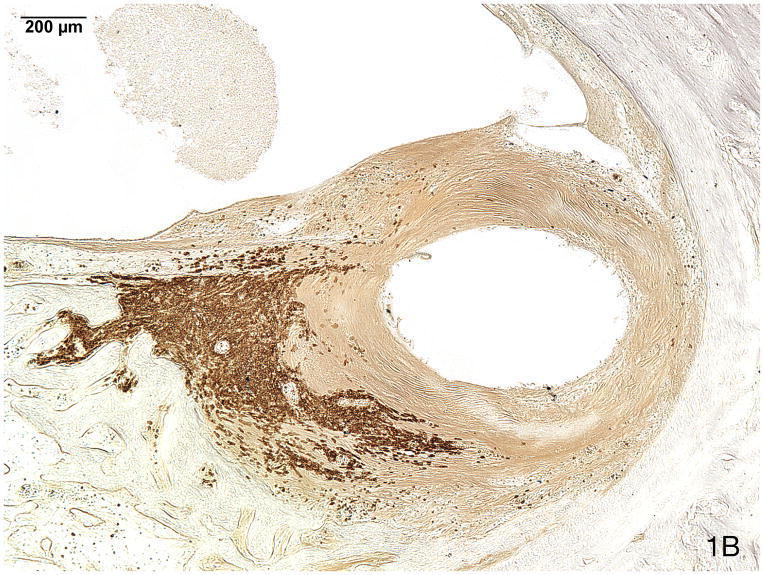
Fig. 1.
Fig. 1A. Basal turn of case #5 (Table). This 91 year old man with a clinical diagnosis of bilateral Meniere’s syndrome underwent a cochlear implant in the left ear at age 81 using a Nucleus 22® device. The NU-6 word score was 12%, as recorded at age 82 years. The electrode track (ET) was present in the scala tympani and was surrounded by a fibrous sheath (FS) and an infiltrate of lymphocytes (L) (H&E staining).
Fig. 1B. Immunostaining (anti-CD20) identified the majority of these lymphocytes as B cells.
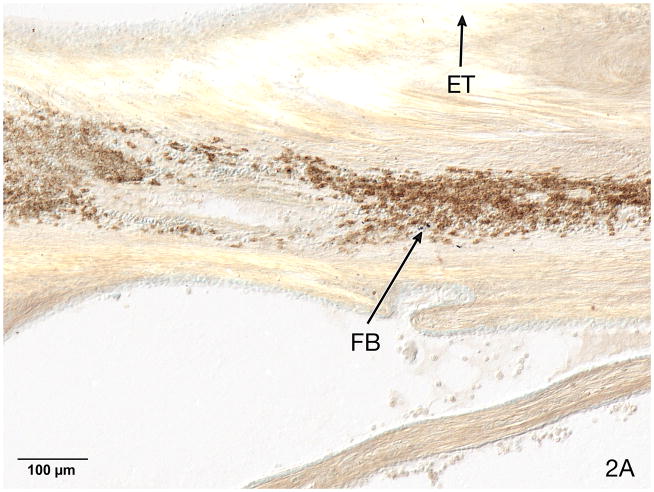
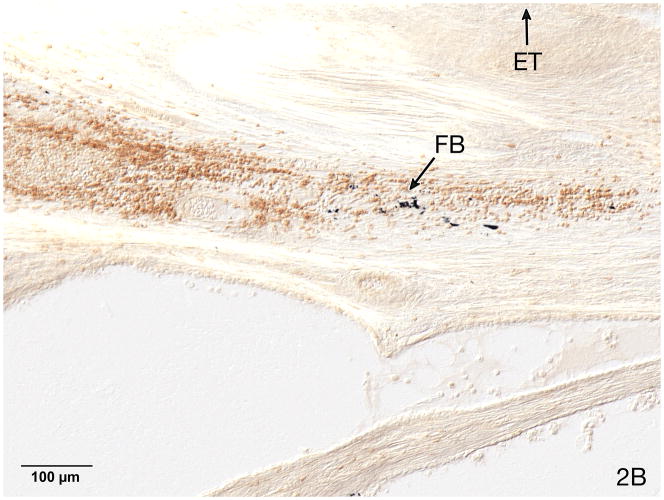
Fig. 2.
Fig. 2A. Basal turn of case #3. This 76 year old woman with a familial hearing loss underwent left cochlear implantation at age 59 years using a Nucleus 22® device. She was a poor implant user and scored 0% on an NU-6 word list at age 50. A B-cell lymphocytic infiltrate was identified using anti-CD20 immunostaining near the electrode track (ET). Opaque particulate foreign body material (FB) was seen within the inflammatory infiltrate.
Fig. 2B. On a semi-serial section a co-population of T-cells was identified using anti CD-3 immunostaining. In addition, opaque particulate foreign body material (FB) was seen within the inflammatory infiltrate.
A pure infiltrate of either B- or T- cells was not found in this small series.
The finding of opaque particles consistent with foreign body material was common in areas of lymphocytic infiltrate (Figs. 2A–B).
Macrophages and Foreign Body Giant Cells
The electrode array was frequently lined by multinucleated foreign body giant cells (Figs. 3 and 4) at the interface between the fibrous sheath and the electrode track. Foreign body giant cells were immunopositive using a CD-68 antibody (Fig. 4).
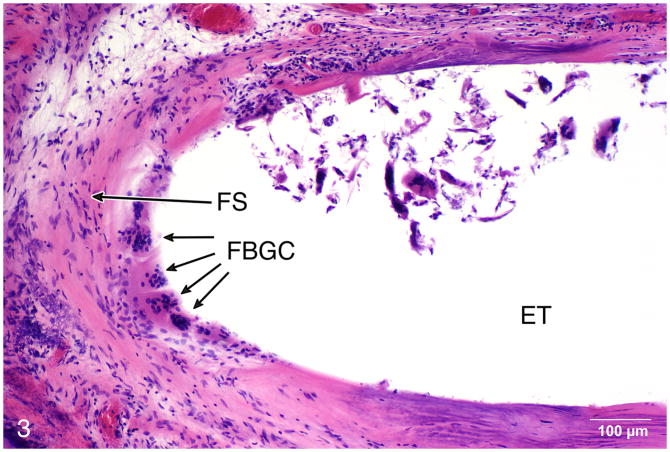
Fig. 3.
Case #4, right ear. This 68 year old man sustained bilateral hearing loss due to basilar skull fracture at age 46 and underwent bilateral cochlear implantation on the right using a Nucleus 22® device at age 47, and on the left a Nucleus Freedom® device at age 64. The patient’s word recognition score was poor, with HINT scores of 0% in both ears at age 66. Multinucleated foreign body giant cells (FBGC) were commonly located in the plane between the fibrous sheath (FS) and the electrode itself (ET) (H&E staining).
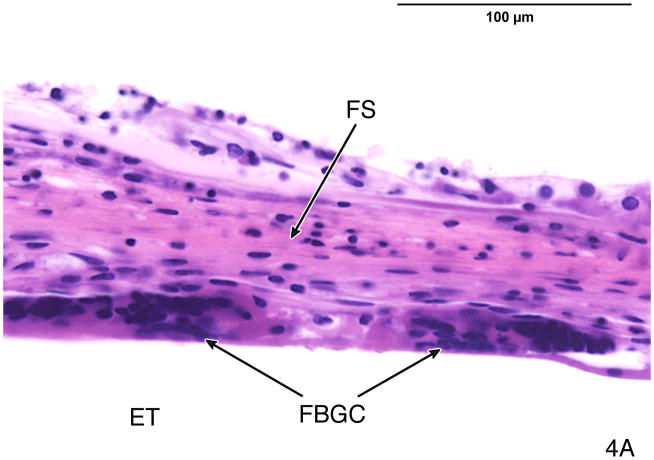
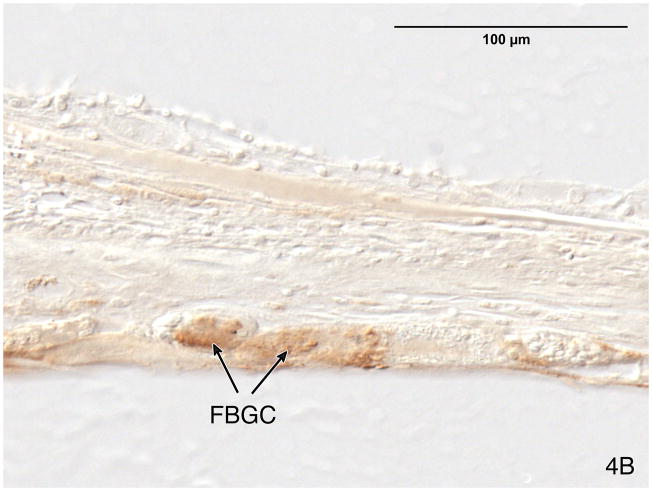
Fig. 4.
Fig. 4A. The fibrous sheath surrounding the electrode track in case #4, left ear. There were multinucleated foreign body giant cells (FBGC) between the fibrous sheath (FS) and the electrode track (ET).
Fig. 4B. The foreign body giant cells (FBGC) were immunostained using anti CD-68 antibody.
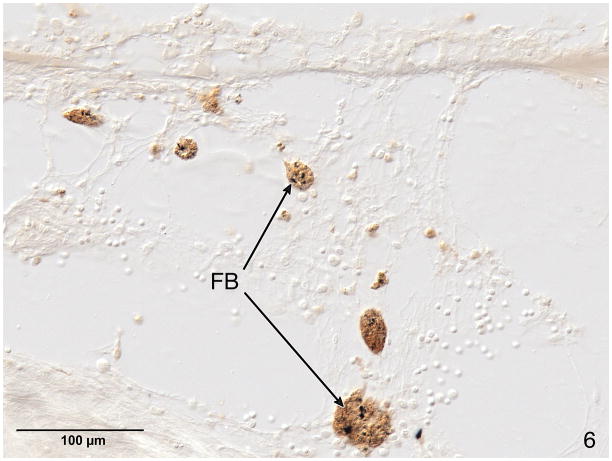
Macrophages were also stained with CD-68 antibody and were located in the fibrous soft tissue of the sheath surrounding the electrode track. These macrophages often contained opaque particles consistent with phagocytized foreign material (Fig. 5 and 6).
Fig. 5.
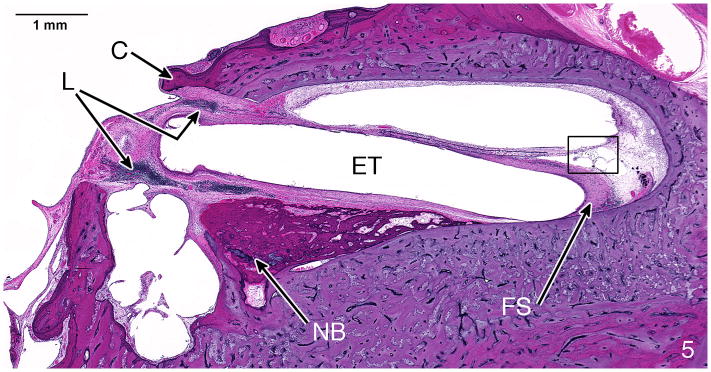
H&E staining of case #4, left ear. Near the electrode track (ET) at the cochleostomy (C) were new bone formation (NB), a lymphocytic infiltration (L) and a fibrous sheath (FS) surrounding the electrode. The boxed area in this figure is shown at higher magnification in Figure 6.
Fig. 6.
Anti CD-68 immunostaining of macrophages in the loose areolar tissue surrounding the fibrous sheath of the electrode track in the basal turn of case #4, left, shown at lower magnification in Fig. 5. These macrophages contained phagocytized opaque particulate foreign material (FB).
Energy Dispersive X-ray Spectroscopy (EDS-SEM)
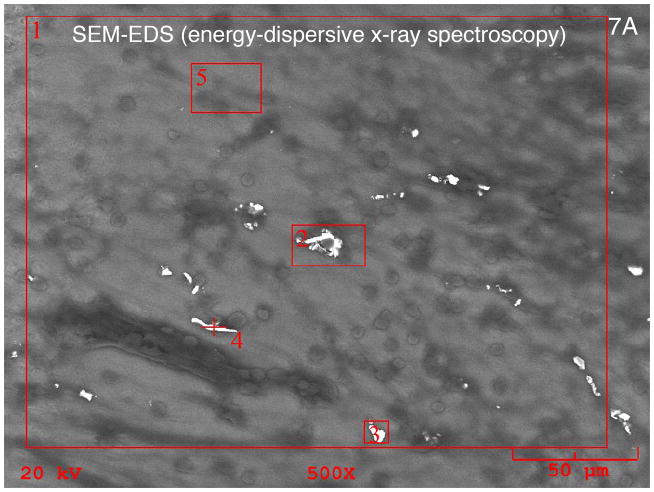
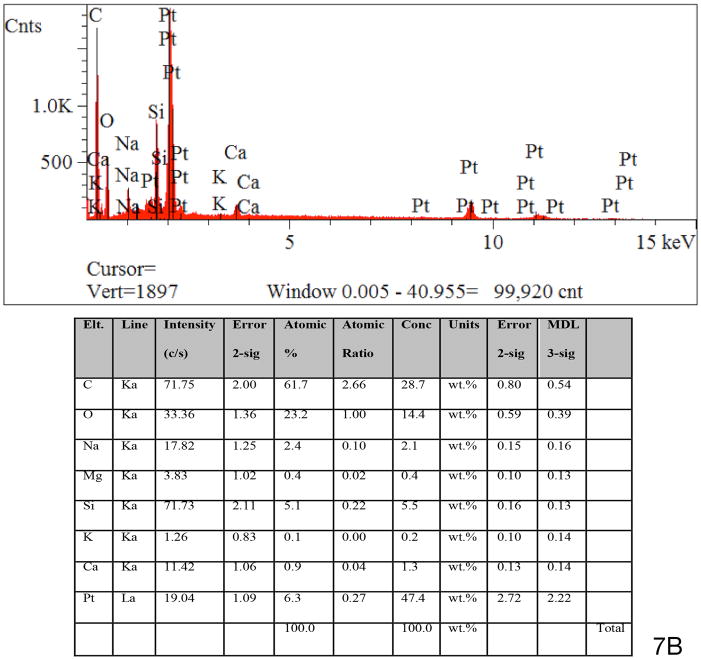
Energy dispersive X-ray spectroscopy of the particulate material found in the fibrous sheath surrounding the cochlear implant electrode was consistent with platinum as the foreign body (Figs. 7A and 7B). Using the EDS system, specific areas of interest were located and analyzed. The EDS system provides a spectrum showing the elements present in each identified area but also reports elemental concentration and weight percent. In this case the concentration is not directly useful but provides a guide to the amount of a given element (platinum in this case) and the system’s own confidence in the measurement of that element. Confidence indication is provided in an error indication and a minimum detection limit (MDL). In some cases trace amounts of platinum may have been reported, but these were below the systems own MDL indicator. In other cases particulate structures were found with platinum in excess of 50% by weight.
Fig. 7.
Fig. 7A. Targeting photomicrograph for EDS-SEM (energy dispersive x-ray spectroscopy) of the particulate material seen surrounding the electrode sheath in case #3. This 76 year old with bilateral progressive hearing loss of unknown etiology starting at age 10 years underwent a left cochlear implant using a Nucleus 22® device at age 59 years. Word recognition score (NU-6 word score) was 0% at age 50 years.
Fig. 7B. The spectroscopy findings were consistent with platinum as the foreign material seen. See Materials and Methods (pg 4), and Results (pg 5) for more complete description of technique.
SEM
Scanning electron microscopy of the electrode removed at the time of histologic preparation demonstrated cellular debris on the surface of the electrode but also evidence of pitting and cracking presumably due to corrosive changes in vivo (Figs. 8 and 9).
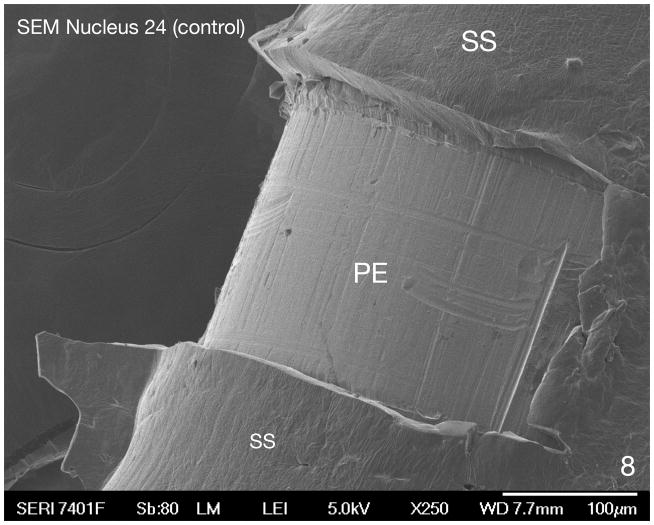
Fig. 8.
SEM of a control Nucleus 24® electrode array (never implanted), near the tip of the electrode. The platinum electrode (PE) can be seen surrounded by a silicone sheath (SS). The surface of the platinum electrode is smooth.
Fig. 9.
SEM of a platinum electrode of a Nucleus 24® device extracted prior to histologic preparation in case #4, left. This electrode had been implanted 4 years before death. Pitting of the platinum electrode (arrows) was consistent with in vivo corrosion.
Discussion
An inflammatory response to the presence of a cochlear implant has been described in both animals (Shepherd RK et al 1984) and in the human (Bertuleit et al, 1999; Kronenberg et al., 2001; Nadol et al., 2004; Kunda et al., 2006; Migirov L et al., 2007; Ho et al., 2007, Doherty et al., 2004; Nadol et al., 2008; O’Leary SJ, et al., 2013; Seyyedi et al., 2014). The most typical biologic response, both in the animal and in the human seems to be a lymphocytic infiltrate and the development of a fibrous sheath encasing the electrode array (Shepherd et al., 1984; Nadol et al., 2004). However, in the human, cases with a more severe response resulting in extrusion of the electrode outside the cochlea (Ho, et al., 2007, Nadol et al., 2008) have been described. The etiology of this biologic response has been attributed to foreign body reaction (Bertuleit et al., 1999; Kronenberg et al., 2001, Migirov et al., 2007; Nadol et al., 2008) with the silicone as the prime suspect as an antigen (Kunda et al., 2006; Puri S et al., 2005; Nadol et al., 2008). The prevalence of a biologic response to the presence of a cochlear implant in the human has been estimated to be in excess of 75 percent of patients (Nadol et al., 2008; Seyyedi et al., 2014). A foreign body or hypersensitivity response has been described after implantation of a variety of other prostheses containing silicone including skin expanders (Maturi et al., 1991), implants for arthroplasty (Kistler et al., 2005); cardiac pacemakers (Maushagen et al., 1994), cerebrospinal fluid shunts (Snow et al., 1989; Jimenez et al., 1994), breast implants (Busch, 1994) and endolymphatic subarachnoid shunt (Pulec JL, 1998). In addition, a biologic response to the presence of nonpolymeric implant material including metal and ceramic has been described (Anderson JM et al., 2008), including platinum (Chan et al 2015).
Particulate material consistent with platinum has been previously observed in a long term human implant recipient (Clark et al, 2013, Clark et al 2014).
The current report describes an exuberant infiltration of type B and type T lymphocytes, foreign body giant cells and macrophages in proximity to the electrode track. In addition, opaque foreign body material was seen in the vicinity of the electrode track either in an extracellular location (Fig. 2B) or apparently phagocytized by macrophages (Fig. 6).
Okano T et al (2008) have suggested that macrophages in the inner ear may be derived from circulating monocytes and consist of two subcategories: 1) infiltrating macrophages migrating from the circulation into the tissue and 2) resident tissue macrophages. The existence of mononuclear phagocytes has also been described in the inner ear of animals by Hirose et al. (2005).
The results of energy dispersive x-ray spectroscopy by SEM identified the particulate material as platinum, probably as a result of pitting and corrosion of the platinum electrode plates (Fig. 8 and 9). The physiologic and clinical consequences of a foreign body reaction to components of the cochlear implant are in most cases unknown at least in the short run. However, in some cases failure of the implant has been described (Bertuleit et al., 1999; Kronenberg et al., 2001; Kunda et al., 2006; Ho et al., 2007; Migirov et al., 2007; Nadol et al., 2008).
In one study, (Groothuis J, et al., 2014) it was suggested that a foreign body response to the presence of stimulating electrodes in the brain could result in electrical malfunction of the electrode and neuronal loss.
There is evidence in an animal model (O’Leary SJ et al., 2013) that the extent of the tissue reaction seen within the cochlea and the presence of foreign body giant cells and new bone formation was negatively correlated with residual hearing following surgical implantation.
In summary, a cellular immune response to the presence of a cochlear implant, including the presence of phagocytized particulate foreign material is common in the human. This seems to result in no significant degradation of performance in most cases. However, in some cases with an exuberant cellular immune reaction, failure or even extrusion of the implant device may occur.
Acknowledgments
We would like to thank Ann Tisdale from the Schepens Eye Research Institute for her expertise, processing, scanning, and photographing of the specimens for the scanning electron microscopy portion of this study.
References
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuleit H, Groden C, Schafer HJ, Leuwer R. Removal of a cochlear implant with chronic granulation labyrinthitis and foreign body reaction. Laryngo-rhino-otologie. 1999;78:304–306. doi: 10.1055/s-2007-996876. [DOI] [PubMed] [Google Scholar]
- Chan W, James C, Sutton-Smith P, Dodd T, Selva D. Histologic evidence of tissue reaction to platinum eyelid chain. Arch Ophthal. 2011;129:1247–1248. doi: 10.1001/archophthalmol.2011.255. [DOI] [PubMed] [Google Scholar]
- Clark GM, Clark JCM, Furness JB. The evolving science of cochlear implants (viewpoint) JAMA. 2013;310:1225–26. doi: 10.1001/jama.2013.278142. [DOI] [PubMed] [Google Scholar]
- Clark GM, Clark J, Cardamone T, Clarke M, Nielsen P, Jones R, Arhatari B, Birbilis N, et al. Biomedical studies on temporal bones of the first multichannel cochlear implant patient at the University of Melbourne. Cochlear Implants International. 2014:1–15. doi: 10.1179/1754762814Y.0000000087. (E Pub) [DOI] [PubMed] [Google Scholar]
- Doherty JK, Linthicum FH., Jr Temporal bone histopathology case of the month: cochlear endosteal erosion with focal osteomyelomas induced by cochlear implantation. Otol Neurotol. 2004;25:1029–30. doi: 10.1097/00129492-200411000-00029. [DOI] [PubMed] [Google Scholar]
- Groothuis J, Ramsey NF, Ramakers GM, Van der Plasse G. Physiologic challenges for intracortical electrodes. Brain Stimul. 2014;7:1–6. doi: 10.1016/j.brs.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the cochlea after acoustic trauma. J Comp Neurol. 2005;489:180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Ho EC, Proops D, Andrews P, Graham J. Unexpected exit of a cochlear implant electrode to the wall of the basal turn of the cochlea-a report on two patients. Coch Implants Inter. 2007;8:162–71. doi: 10.1179/cim.2007.8.3.162. [DOI] [PubMed] [Google Scholar]
- Killer M, Arthur AS, Barr J, Richling B, Cruise GN. Histomorphology of thrombus organization, neointima formation, and foreign body response in retrieved human aneurysms treated with hydrocoil devices. J Biomed Mater Res B: Applied Biomater. 2010;94:486–492. doi: 10.1002/jbm.b.31660. [DOI] [PubMed] [Google Scholar]
- Kistler U, Weiss AP, Simmen BR, Herren DB. Long term results of silicone wrist arthroplasty in patients with rheumatoid arthritis. J Hand Surg (Am) 2005;30:1282–7. doi: 10.1016/j.jhsa.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Kronenberg J, Wolf M, Migirov L, Shapira Y, Aviel-Ronen S, Hildesheimer M. Foreign body reaction to cochlear implant. Oto-rhino-laryngol Nova. 2001;11:207–209. [Google Scholar]
- Kunda LD, Stidham KR, Inserra MM, Roland PS, Franklin D, Roberson JB., Jr Silicone allergy: a new cause for cochlear implant extrusion and its management. Otol Neurotol. 2006;27:1078–1082. doi: 10.1097/01.mao.0000235378.64654.4d. [DOI] [PubMed] [Google Scholar]
- Maturri L, Azzolini A, Campiglio GL, Tardito E. Are synthetic prostheses really inert: preliminary results of study on the biocompatibility of Dacron vascular prostheses and Silicone skin expanders. Int Surg. 1991;76:115–18. [PubMed] [Google Scholar]
- Maushagen E, Reischle B, Simon H. Circumscribed erythema after cardiac pacemaker implantation. Z Kardiol. 1994;83:340–342. [PubMed] [Google Scholar]
- Meyer DR, Bui HX, Carlson JA, et al. Silicone granulomas and dermatomyositis-like changes associated with chronic eyelid edema after silicone breast implant. Ophthal Plast Reconstr Surg. 1998;14:182–8. doi: 10.1097/00002341-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Migirov L, Taitelaum-Swead R, Hildesheimer M, Kronenberg J. Revision surgeries in cochlear implant patients: a review of 45 cases. Eur Arch Oto-rhino-laryngol. 2007;264:3–7. doi: 10.1007/s00405-006-0144-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Otol & Neurotol. 2008;29:1076–1084. doi: 10.1097/MAO.0b013e31818c33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB, Jr, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol & Neurotol. 2004;25:257–262. doi: 10.1097/00129492-200405000-00010. [DOI] [PubMed] [Google Scholar]
- O’Leary SJ, Monksfield P, Kel G, Connolly T, Souter MA, Chang A, Marovic P, O’Leary JS, Richardson R, Eastwood H. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hear Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- O’Malley JT, Burgess BJ, Jones DD, Adams JC, Merchant SN. Techniques of celloidin removal from temporal bone sections. Ann Otol Rhinol Laryngol. 2009 Jun;118(6):435–441. doi: 10.1177/000348940911800606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley JT, Merchant SN, Burgess BJ, Jones DD, Adams JC. Effects of fixative in embedding medium on morphology and immunostaining of the cochlea. Audiol Neuro Otol. 2009;14(2):78–87. doi: 10.1159/000158536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J. Bone marrow derived cells expressing IBA1 are consistently present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008;86:1758–67. doi: 10.1002/jnr.21625. [DOI] [PubMed] [Google Scholar]
- Pepas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- Puri S, Dornhoffer JL, North PE. Contact dermatitis to silicone after cochlear implantation. Laryngoscope. 2005;115:1760–2. doi: 10.1097/01.mlg.0000172202.58968.41. [DOI] [PubMed] [Google Scholar]
- Seyeddi M, Nadol JB., Jr Intracochlear inflammatory responses to cochlear implant electrodes in the human. 2014 doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Webb RL, Clark GM, et al. Implanted material tolerance studies for a multi-channel cochlear prosthesis. Acta Otolaryngol Suppl. 1984;411:71–81. [PubMed] [Google Scholar]
- Snow RB, Kossovosky N. Hypersensitivity reactions associated with sterile ventriculo-peritoneal shunt malfunction. Surg Neurol. 1989;31:209–14. doi: 10.1016/0090-3019(89)90119-5. [DOI] [PubMed] [Google Scholar]