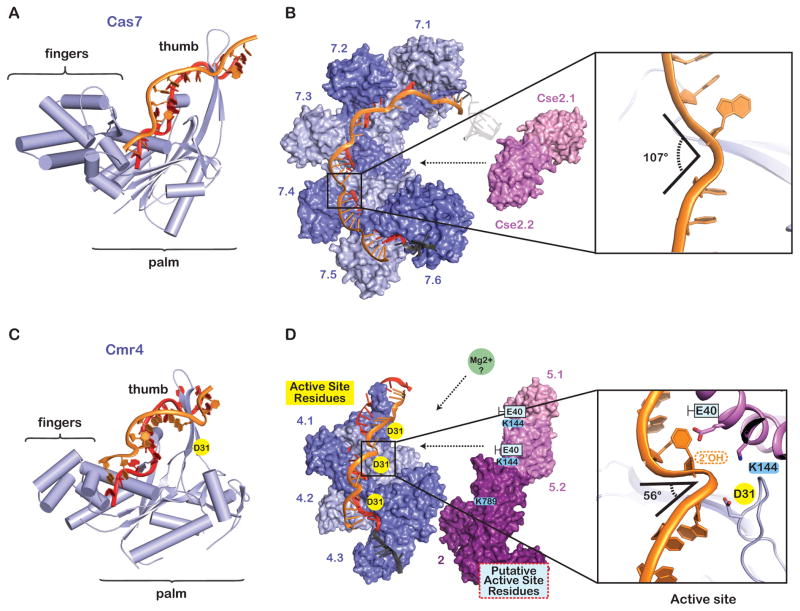
Figure 2. The helical backbone is a versatile architectural platform with mechanistic plasticity.
(A) The Cas7 protein from E. coli (Type I-E) is shaped like a right hand. The palm of Cas7 binds the crRNA through non-sequence specific interactions and the thumb pierces the crRNA/DNA duplex (PDB 4QYZ). (B) The Cascade backbone is composed of six interwoven Cas7 proteins (Cas7.1–7.6). The Cas7 thumbs distort the backbone geometry of the single-stranded DNA target, introducing ~107-degree kinks at 6-nucleotide intervals. (C) The Cmr4 protein from A. fulgidus is a Cas7 family protein with a “right hand” morphology. The crRNA is non-specifically bound by the palm and the thumbs pierce the crRNA/target duplex at 6-nucleotide intervals (PDB 3X1L). (D) The catalytic residue (D31) on Cmr4 is adjacent to ~56-degree kinks in the phosphate backbone. Conserved residues on Cmr5 (K144) and Cmr2 (K789) interact with the kinked out phosphate, and the side chain of a non-conserved residues (e.g. Cmr5E40) nudges the kinked bases towards the major groove of the adjacent duplex.