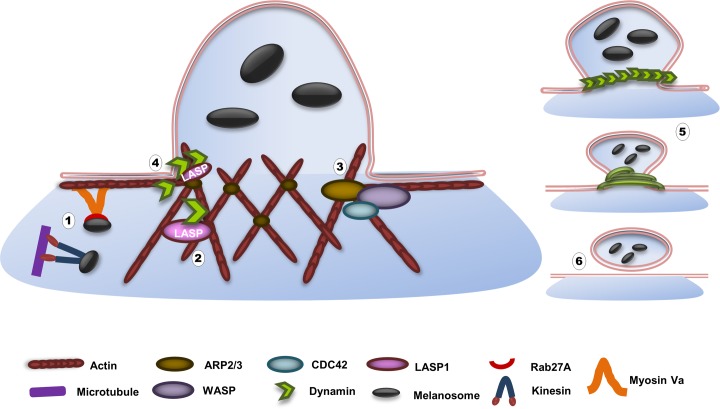
Fig 10. Proposed model for LASP1 involvement in actin-dynamin- mediated melanosome vesicle scission at melanocyte dendrite tips.
In anterograde melanosomal transport mature melanosomes move along microtubules by means of the motor protein kinesin and are transferred towards the cell periphery. Once at the periphery, track switching from microtubules to actin filaments occurs, a process mediated by Rab27A and molecular motor protein myosin Va (1). In this fashion microtubule, actin filaments and motor systems co-operate to promote melanosome transport and retention of the organelle in peripheral dendrites. LASP1 and dynamin are present at the actin mesh at the plasma membrane. LASP1 binds to actin and dynamin through its nebulin repeat and SH3 domain, respectively. Dynamin exists as a dimer and binds to actin through the stalk region (2). WASP- and CDC42-mediated activation of ARP2/3 lead to the branched polymerization of actin filaments towards the release site. Actin, together with other motor proteins, pushes the plasma membrane and enhances membrane invagination (3). As an adaptor/scaffolding protein, LASP1 recruits and positions dynamin at the tubular membrane (4). Subsequent polymerization of dynamin around the membrane in a helical manner and GTP hydrolysis results in membrane constriction and melanosome vesicle scission at dendrite tips (5 and 6).