Abstract
Hypercapnia is known to have immunoregulatory effects within the lung. Cell culture systems demonstrate this in both macrophages and alveolar cell lines, suggesting that alveoli are affected by changes in CO2 levels. We hypothesized that hypercapnia would also modulate human bronchial epithelial cell immune responses. Innate immune responses to Pam3CSK4 (TLR2 ligand), LPS (TLR4 ligand) and a complex innate immune stimulus, an extract from the organic dust of swine confinement barns (barn dust extract or BDE), were tested in a human bronchial epithelial cell line, BEAS-2B. Both TLR ligands showed a decrease in IL-6 and IL-8 production, and an increase in MCP-1 in response to elevated CO2 indicating an enhancement in cytokine production to hypercapnia. This change was not reflected in expression levels of TLR receptor RNA which remained unchanged in response to elevated CO2. Interestingly, barn dust showed an increase in IL-6, IL-8 and MCP-1 response at 9% CO2, suggesting that elevated CO2 exerts different effects on different stimuli. Our results show that airway epithelial cell immune responses to barn dust respond differently to hypercapnic conditions than individual TLR ligands.
Keywords: Hypercapnia, TLR, LPS, Barn Dust, Carbon Dioxide, Lung, Bronchial Epithelium
Introduction
Workers in concentrated animal feeding operations (CAFOs) face a number of health problems over the course of their careers, many of these associated with the lungs due to organic dust exposure. These problems can include chronic bronchitis, COPD, and asthma (Just et al., 2009, Poole and Romberger, 2012). Many of these conditions involve airflow obstruction in the lung, which may result in elevated CO2 within the lung, such as observed in COPD patients (Begin and Grassino, 1991). Studies conducted with organic dusts show that innate immune responses to microbial ligands, such as endotoxin and peptidoglycan, found within these dusts are a major cause of many of these symptoms(Donham et al., 2000,Kirychuk et al., 2006,Poole et al., 2007).
CAFOs also contain elevated levels of gasses, including CO2, ammonia, and hydrogen sulfide (Stinn et al., 2012,Dong et al., 2009). The CO2 levels found in these facilities may be up to 0.7% higher than ambient air (Dong et al., 2007), confounding the impact on the seasoned workers who must also contend with airway obstruction developing over time. Shorter common CO2 exposures such as smoking may result in CO2 exposures ranging up to 12.5% (Norman, 1977). The combination may lead to significant changes in CO2 levels deep within the lungs. Many cell culture studies to date study higher CO2 test levels than are found occupationally or environmentally (Abolhassani et al., 2009,Takeshita et al., 2003,Gates et al., 2013,Vaporidi et al., 2005,O'Toole et al., 2009). Examining how varying CO2 levels can induce immune changes in the lung is vital to determining whether exposure to environmental CO2 affects innate immunity.
In addition to CAFO workers, hypercapnia also occurs clinically in other pulmonary patients such as in acute respiratory distress syndrome (ARDS). Work in ARDS studies suggests that permissive hypercapnia in ventilated patients may have beneficial effects in reducing lung inflammation (Laffey and Kavanagh, 1999). Some link these effects not to reduced mechanical stretch of the lungs, but to elevated CO2 (Curley et al., 2010). In contrast, others show increased inflammation due to hypercapnia (Nichol et al., 2009), and animal studies have yielded conflicting results, with some showing reductions in cytokines, while others show increased inflammation in other cell systems (Rai et al., 2004,Abolhassani et al., 2009,Sinclair et al., 2002).
Hypercapnia cell culture studies with cells found in the alveolar space also yield conflicting results. Alveolar epithelial cells show increased inflammatory cytokine production (Abolhassani et al., 2009); whereas, a differentiated monocyte cell line shows reduced IL-6 production (Wang et al., 2010). Hypercapnia appears to primarily affect cytokine production, as cell viability and cell cycle progression appear unaffected (Vaporidi et al., 2005), though this is also unresolved (Vohwinkel et al., 2011). One possible reason for these conflicting results could be that different cell types within the lung respond quite differently to the same hypercapnic conditions, so that while inflammation is reduced in the alveolar space, it may be increased elsewhere. Differences in exposure and reporting systems may also play a role. As airway epithelial cells remain unstudied, we tested the human bronchial epithelial BEAS-2B cell line for changes in inflammatory response to common toll-like receptor (TLR) ligands and organic barn dust extract using a range of CO2 levels, ranging from standard cell culture conditions to lower levels shown to induce hypercapnic changes (5%-9%). We hypothesized that similar to alveolar epithelial cells, bronchial epithelial cells would show an increase in inflammatory cytokine production. TLR2 (synthetic triacylated lipoprotein; Pam3CSK4) and TLR4 (lipopolysaccharide; LPS) ligands were chosen given the role both TLRs have in barn dust immunogenicity (Poole et al., 2007,Dosman et al., 2006) and because others show that hypercapnic effects alter NF-κB signaling, through which both of these receptors signal (Takeshita et al., 2003). We further examined at what level above standard culture conditions (5% CO2) any possible response to elevated CO2 could be measured. We show that not only do bronchial epithelial cells produce less IL-6 and IL-8 but also more MCP-1 to TLR stimulation ligands under hypercapnic conditions. These changes are observed at as little as a 2% increase in CO2 over standard 5% culture conditions. In contrast, exposure to barn dust under hypercapnic conditions resulted in an increase in IL-6, IL-8 and MCP-1, showing that response to different stimuli are affected in different ways by hypercapnia.
Materials and Methods
Cell Culture System
BEAS-2B, a human bronchial epithelial cell line, were purchased from American Type Culture Collection (ATCC, Manassas, VA), cultured and grown in Vitrogen-coated (Invitrogen, Carlsbad, CA) tissue culture flasks at 37°C and 5% CO2 in 1:1 LHC9:RPMI supplemented with penicillin and streptomycin (Gibco, Grand Island, NY). Monolayers were harvested by treatment with trypsin for 10 minutes at 37°C. 100μl trypsin inhibitor was added (Sigma, St. Louis, MO) to inactivate trypsin, resuspended in media and centrifuged to wash cells and replace media and counted. Normal human bronchial epithelial cells (NHBE) were similarly cultured using serum free bronchial epithelial basal media (Lonza, Walkerville, MD). Media was tested at normal and 9% CO2 levels for changes in pH by RapidPoint 500 blood gas analyzer (Siemens, Tarrytown, NY). Monolayers were harvested by treatment with TrypLE Express (Gibco, Denmark) for 10 minutes at 37°C. 100μl trypsin inhibitor was added to inactivate trypsin, resuspended in media and centrifuged to wash cells and replace media and counted.12-well tissue culture plates were coated for a minimum of 10 minutes with 1% Vitrogen before adding 0.3×106 cells in 800 μl to each (approximately 70% confluence). Cells were allowed to attach overnight then washed with PBS, pH 7.4 and fresh media added at time of treatment. After treatment with TLR ligands or BDE, cells were cultured at 37°C at 5%-9% CO2 in 1% increments in a standard cell culture incubator (Model MCO-19AICUV-PA; Sanyo, Wood Dale, IL,). The same incubator was used for all trials and calibrated using a fyrite analyzer (Bacharach, New Kensington, PA). A control using acidified (10mM HCL, media pH 7.0) media at 5% CO2 levels was also performed to control for effects of CO2 induced media acidosis.
Immunostimulatory ligands
Ligands for two of the most commonly studied TLRs, Pam3CSK4 (TLR2) and LPS (TLR4), were administered to cells at either 10 ng/ml or 100EU respectively and immediately exposed to cell culture CO2 conditions as various levels. These doses were determined to be stimulatory, but not maximal (results not shown). Both ligands were diluted in LHC9:RPMI cell culture media.
BDE extracts were prepared from combined settled dust samples taken from two separate swine confinement facilities. Dust extracts were prepared as previously described (Romberger et al., 2002). Briefly, dust (1 g) was mixed with 10 ml HBSS without calcium. This mixture was incubated for 1 hr at room temperature before 10 min centrifugation, with the media being decanted and sterile-filtered for use, for a final concentration of approximately 0.105g/ml dust. Extracts were used at a concentration of 5% v/v of culture per well (40 μl) or about 0.005g/ml dust.
Lactate Dehydrogenase Assay
Lactate dehydrogenase was measured in media samples to determine cell viability using the Lactate Dehydrogenase Activity Assay Kit (BioVision, Milpitas, CA) according to instructions provided. Media samples from cultured cells were tested for 24 hr exposure at all CO2 levels and treatments (n=3 per group). No significant changes in cell death were noted as a result of treatments.
ELISAs
Cell culture media was collected and tested using a sandwich ELISA. IL-6, IL-8, and MCP-1 were tested in duplicate and quantified as per manufacturer's instructions (R&D Systems, Minneapolis, MN). Plates were read using an Epoch microplate reader (BioTek, Winooski, VT). Values given are reported in pg/ml.
Chemokine Array Assay
Cell culture media from 24 hr cell culture exposures were sampled equally to obtain a 1 ml sample pool for testing. Media was tested using a semi-quantitative protein microarray according to manufacturer's instructions (Ray Biotech, Norcross, GA). Sample binding and secondary antibody binding steps were incubated on arrays overnight at 4°C. Arrays were developed with fluorescent marker and exposed to film (GeneMate Blue Ultra; ISC BioExpress, Kaysville, UT) for approximately 3 sec. Developed film was quantitated via densitometry using ImageJ software (http://rsbweb.nih.gov/ij/) and compared to each other by mathematically equalizing control spots (lower right duplicate) on different arrays to one another.
NF-kB Translocation/Binding
Cells were cultured onto 96-well plates and reverse transfected using the Cignal Vector Reporter for NF-kB (SABiosciences; Valencia, CA) as per manufacturer's protocol using lipofectamine 2000 (Invitrogen; Grand Island, NY). Cells were treated with 5% BDE for 24 hr and incubated at 37°C at 5% or 9% CO2. Cells were harvested using Promega Dual-Glo Luciferase Reagent (Promega; Madison, WI). Firefly and renilla luciferase activity was measured using a VICTOR 3V plate reader (Perkin Elmer; Waltham, MA).
Real-time RT-PCR
cDNA synthesis was done using the Taqman reverse transcription kit (Applied Biosystems, Branchburg, NJ) with 100 ng of template RNA purified from cells using the PARIS kit (Ambion, Austin, TX). cDNA synthesis (RT-PCR) reactions contained the following reagents: 1X TaqMan RT buffer, 5.5 nM MgCl2, 500 μM of each dNTP, 2.5 μM random hexamers, 0.4 U/μl RNase inhibitor, and 1.25 U/μl MultiScribe reverse transcriptase. Samples were incubated at 25°C for 10 min, then 48°C for 30 min, and 95°C from 5 min in a thermocycler (MJ Mini; Bio-Rad, Hercules, CA). Real-time PCR reactions consisted of 1X TaqMan Master Mix along with human IL-6, IL-8, and TLR1, 2, 4, 5, 6 primers and probes (Applied Biosystems, Branchburg, NJ; Hs03929033_u1, Hs01567913_91 ,Hs00413978_m1, Hs00152932_m1, Hs00152939_m1, Hs00152825_m1, and H200271977_s1 respectively). Ribosomal RNA was used as an endogenous control. PCR was completed in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Reactions were carried out for 2 min at 50°C, 10 min at 95°C, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min each. All reactions were carried out in duplicate. For relative comparison of TLR2 to the ribosomal RNA endogenous control, we analyzed cycle threshold (C1) value of real-time PCR data with ΔΔCt method (Livak and Schmittgen, 2001).
Statistical Analysis
Results are given as means, and error bars denote SEM. Comparisons between groups were done using 1-way ANOVA. All calculations were done using GraphPad Prism v5.0 (GraphPad Software Inc., La Jolla, CA).
Results
Cytokine response
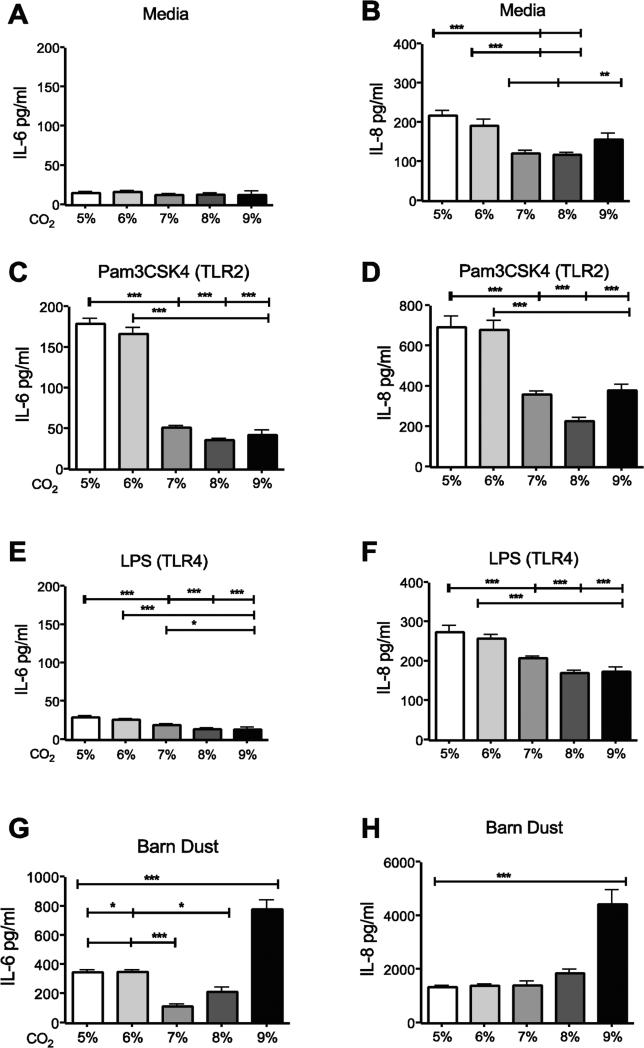
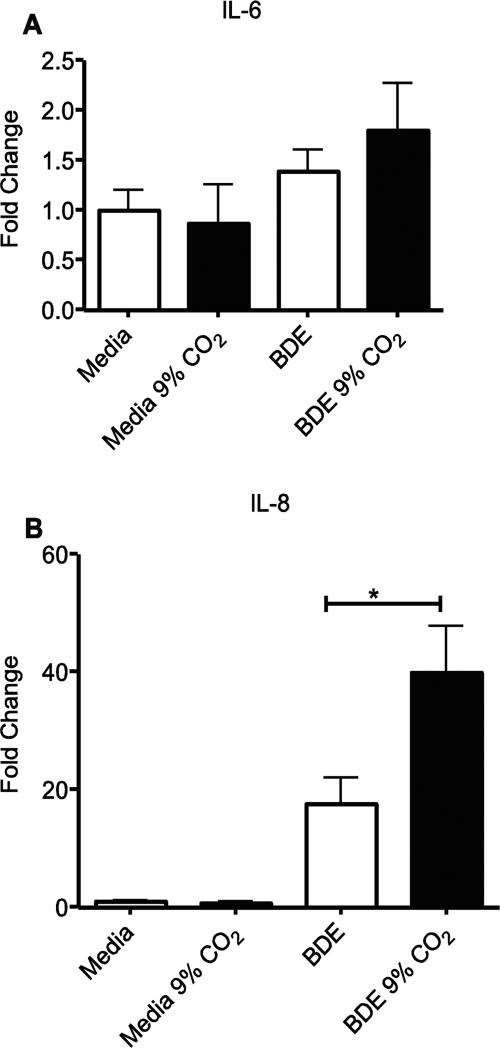
To determine effects of hypercapnia on inflammation, we looked at IL-6 and IL-8, an important pro-inflammatory cytokine (IL-6) and chemokine (IL-8) produced in the lung. We found that in unstimulated BEAS-2B cells treated with media alone (control) there was no significant change in levels of IL-6 (Fig. 1A) as compared to standard culture conditions (5% CO2); however, IL-8 was reduced at 7-9% CO2 levels compared to 5% (Fig. 1B). The slight increase observed at 9% CO2 was not significant (Fig. 1B). A similar pattern of decrease at higher CO2 levels was seen with Pam3CSK4 (Fig. 1C, 1D) and LPS stimulation (Fig. 1E, 1F). The response was different for barn dust extract-stimulated cells. In this case, elevated CO2 had no effect on cytokine or chemokine expression until 9% CO2 was used, at which point the effect was stimulatory, not inhibitory (Fig. 1G, 1H).
Figure 1.
Elevated CO2 reduces the expression of IL-6 and IL-8 in BEAS-2B cells. BEAS-2B cells were treated for 24 hr with either (A,B) media, (C,D) TLR2 ligand (Pam3CSK4, 10ng/ml), (E,F) TLR4 ligand (LPS, 100EU), or (G,H) 5% v/v barn dust, and incubated at several levels of CO2 above standard culture conditions (5% CO2) in 1% increments from 5%-9%. Media was measured for IL-6 and IL-8 by ELISA assay. Both TLR ligands show significant drops in both cytokines by 7% CO2, and IL-8 was reduced in media control at the same CO2 level, however IL-6 was also reduced by CO2 exposure alone. Barn dust however showed a significant increase in both cytokines at 9% CO2. Mean results are given as concentrations of cytokine per well. Statistical significance * P<0.05, ** P<0.01, *** P<0.001 (n=9).
As acidosis is a feature of hypercapnia, we tested media pH after 24hr exposure at 5% and 9% CO2. Media at 5% had a pH of 7.27, whereas that at 9% it was at a pH of 7.07. As this is sufficient in some systems to cause detectable immunological changes (Wang et al., 2010) we subsequently tested IL-8 production in BEAS-2B cells with all ligands in acidified (pH 7.0) media to see if this was the case with this cell type. LPS, PAM3CSK4, and barn dust all showed no significant changes to cytokine production (Supplemental Fig 1). Changes in cytokine production were therefore a function of increased CO2, not decreased pH.
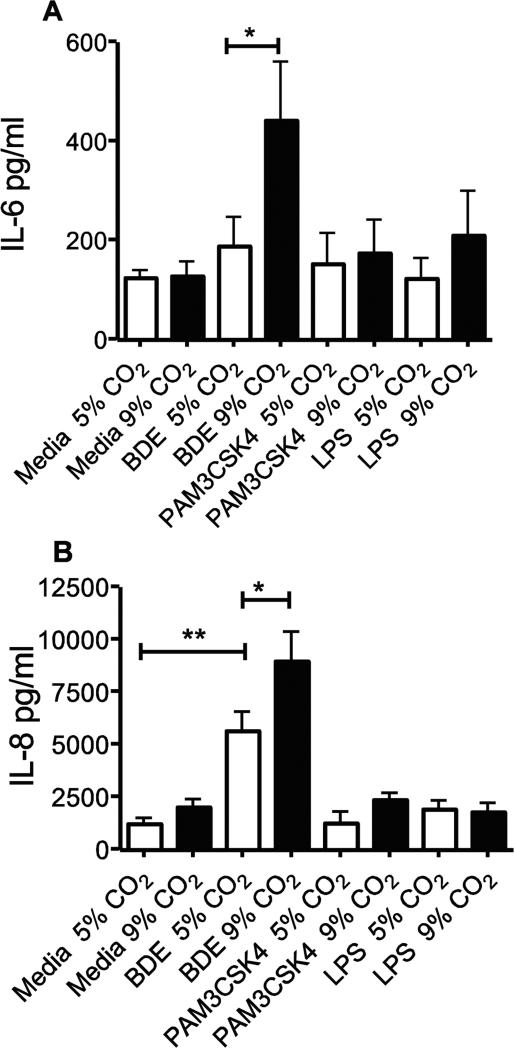
NHBE cells were tested at 5% and 9% CO2 to confirm these results. Identical to what was shown in BEAS-2B cells, BDE induced a significant (p<0.05) increase in IL-6 and IL-8, and BDE + 9% CO2 levels were significantly higher than BDE given at 5% CO2 (p<0.05 both) (Fig. 2A, 2B). The NHBE cells tested however returned poorer levels of IL-6 and IL-8 in response to both LPS and Pam3CSK4 (Fig. 2C, 2D), with no groups being significantly different from media controls.
Figure 2.
Elevated CO2 reduces the expression of IL-6 and IL-8 in NHBE cells. NHBE cells were treated for 24 hr with either media, TLR2 ligand (Pam3CSK4, 10ng/ml), TLR4 ligand (LPS, 100EU), or 5% v/v barn dust, and incubated at 5% or 9% CO2. Media was measured for IL-6 (A) and IL-8 (B) by ELISA assay. Both TLR ligands show no significant changes in both cytokines at either CO2 level. Barn dust however showed a significant increase in both cytokines at 9% CO2. Mean results are given as concentrations of cytokine per well. Statistical significance * P<0.05, ** P<0.01, (n=9).
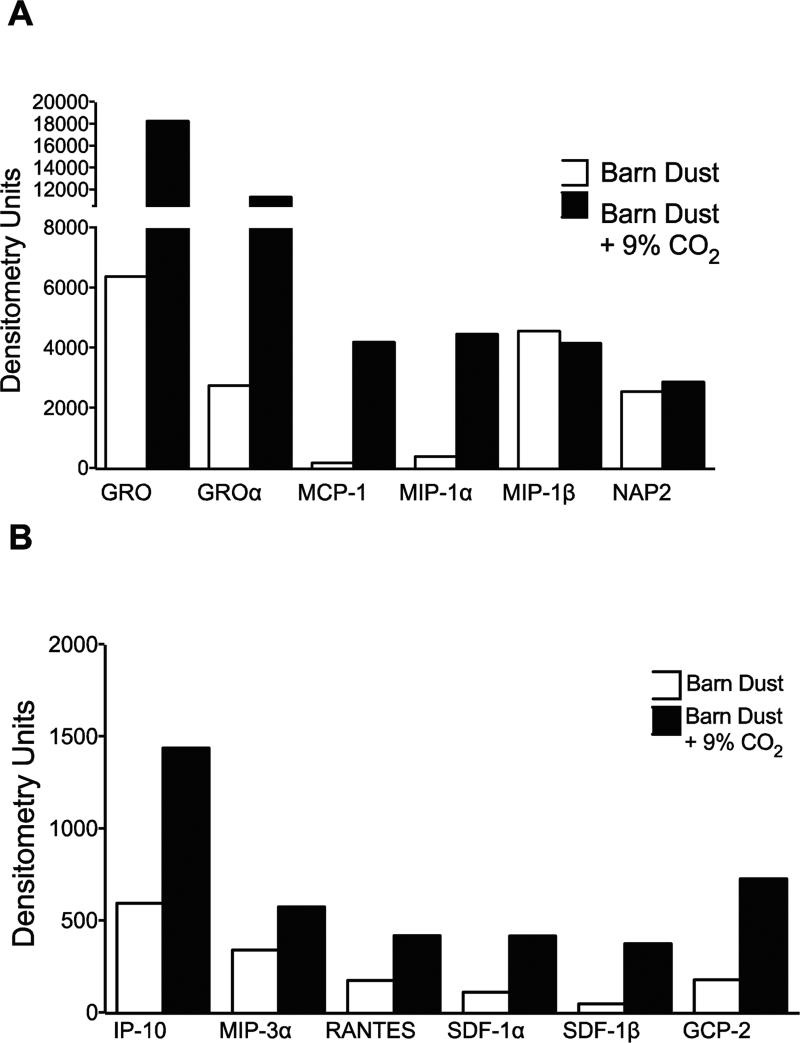
To further examine this effect, media from media or barn dust treated BEAS-2B cells exposed for 24 hours at 5% or 9% CO2 were tested. Nine replicate samples from each treatment were pooled and tested using a chemokine protein array. Statistical analysis was not performed on the pooled samples. Images were quantified by image analysis software and expressed as densitometry units. We showed the increase of a number of chemokines at 9% CO2 (Fig. 3). While many chemokines were present at low levels, several showed greater than 2-fold increases in chemokine expression with elevated CO2 and strong expression such as GRO, GRO-α, MCP-1, MIP-1α, MIP-1β, and NAP2. Of these, only MIP-1β was slightly greater in cells incubated at normal CO2 levels and NAP2 was found at similar levels at both CO2 levels.
Figure 3.
Chemokine array of barn dust exposure to 9% CO2. Media from BEAS-2B cells exposed to barn dust (5% v/v) for 24 hr was pooled from (n=9) separate test samples and tested for chemokine protein expression. Dot blot arrays were exposed to film for 3 seconds, scanned, and quantified using densitometry software. Numbers shown as relative densitometry units. High expression chemokines (>2000 units) (A) and lower expressed chemokines (<2000 units) (B) showed a general pattern of increased production in 9% CO2 incubated cells across all secreted chemokines.
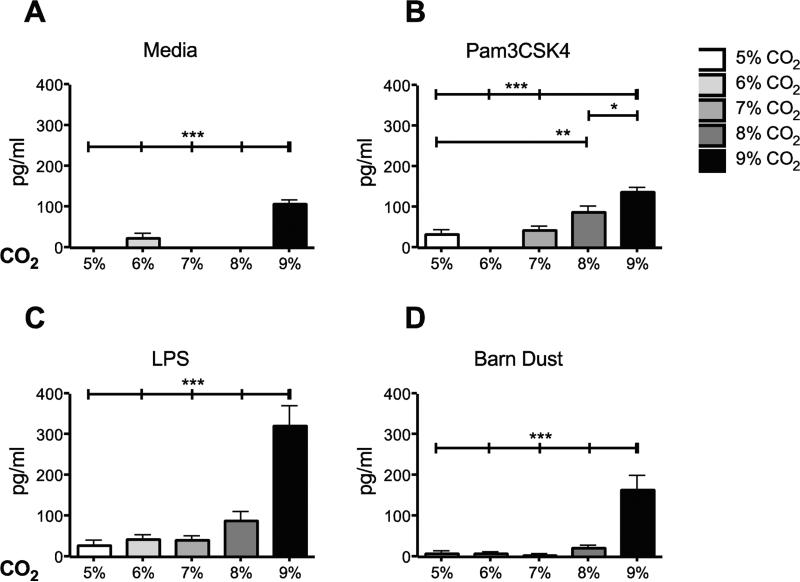
MCP-1 was selected for closer examination given its apparent sensitivity to hypercapnia, its role in inflammation, and possible anti-inflammatory function (Chensue et al., 1996,Leonard and Yoshimura, 1990). Over several trials, the media control group showed a significant induction of MCP-1 at 9% CO2 (Fig. 4A). While barn dust plus 9% CO2 resulted in a significant increase in MCP-1 similar to that seen with IL-8 for barn dust exposed cells (Fig. 4D). The response to TLR ligands was quite different. MCP-1 was increased with Pam3CSK4 (Fig. 4B) and LPS plus CO2 (Fig. 4C). These elevated MCP-1 levels were apparent in cells stimulated with Pam3CSK4 at as little as 7% CO2, with a steady increase to 9%. LPS induction of MCP-1 was significantly altered at 9% CO2, but the change was greater than seen for Pam3CSK4. No detectable levels of MCP-1 were discernable in NHBE cells however for any treatment or condition. Thus we show clear changes in cytokine expression in BEAS-2B cells to hypercapnia to two TLR ligands and BDE.
Figure 4.
Elevated CO2 increases the expression of MCP-1 in BEAS-2B cells. BEAS-2B cells were treated for 24 hr with either (A) media, (B) TLR2 ligand (Pam3CSK4), (C) TLR4 ligand (LPS), or (D) 5% v/v barn dust, and incubated at several levels of CO2 above standard culture conditions (5% CO2). Media was measured for MCP-1 by ELISA assay. Media, TLR ligands, and barn dust show significant increases in MCP-1 at different CO2 levels, with the greatest increase at 9% CO2. Mean results are given as concentrations of cytokine per well. Statistical significance * P<0.05, ** P<0.01, *** P<0.001 (n=9).
To determine if the changes to cytokine production were at the transcriptional or translational level we tested mRNA expression in BEAS-2B cells. While IL-6 expression was not significantly increased a trend to increased expression at 9% CO2 was apparent (Fig. 5A-B). IL-8 mRNA expression was significantly increased by BDE exposure (p<0.0001), and levels in BDE exposed cells cultured at 9% CO2 were significantly higher still than those at 5% CO2 (p<0.05).
Figure 5.
Elevated CO2 increases IL-8 mRNA expression in BEAS-2B cells. RNA was purified from BEAS-2B cells treated for 24 hr with media alone or 5% v/v barn dust and incubated at 5% or 9% CO2. RT-PCR of samples was quantified and fold change from standard calculated. There was a significant elevation of IL-8 mRNA in response to barn dust and this was significantly elevated at 9% CO2. Statistical significance * P<0.05, **** P<0.0001. (n=6). No significance was seen for IL-6, though there was a trend to increased IL-6 mRNA similar to what was seen with IL-8.
TLR Receptor
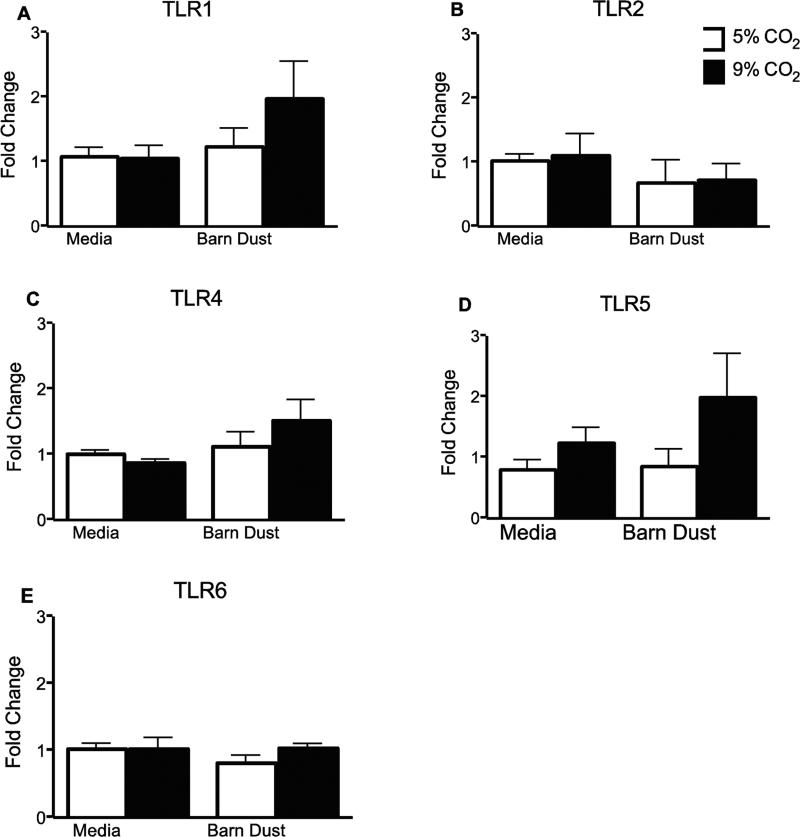
TLR receptor expression was examined as a possible reason for changes in cytokine response due to hypercapnia. BEAS-2B cells were examined for expression of TLRs1, 2, 4, 5, and 6 after 24 hr treatment with barn dust or media at 5% and 9% CO2. No significant changes were detected between control and elevated CO2 for either media or BDE, or between media and BDE at comparable for any TLR examined (Fig. 6A-E).
Figure 6.
TLR receptor expression. RNA was purified from BEAS-2B cells exposed to 5 or 9% CO2 plus media or 5% v/v barn dust for 24 hrs. RT-PCR of samples was quantified and fold change from standard calculated. No significant changes were noted in any treatments (n=9).
NF-kB Activity
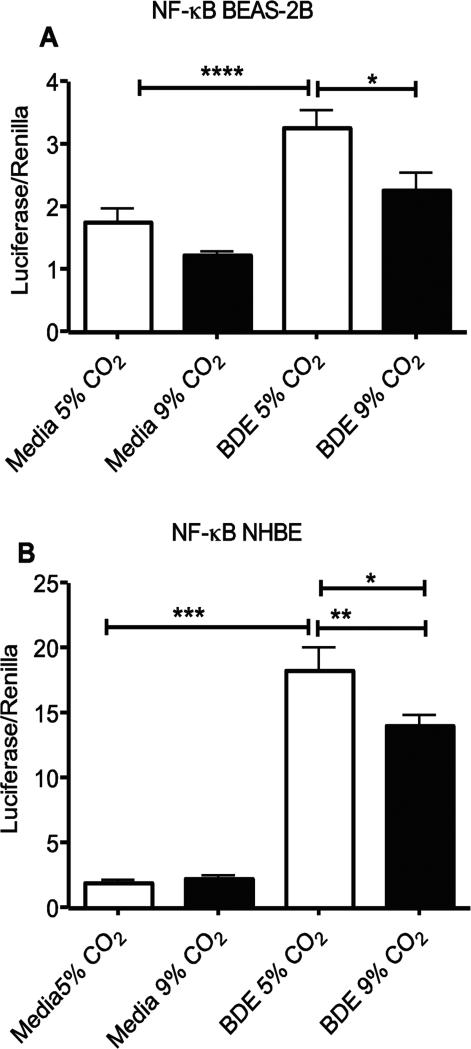
An increase in NF-kB translocation to the nucleus is common in inflammation but has been shown by some to be decreased under hypercapnic conditions (Takeshita et al., 2003,Cummins et al., 2010). BEAS-2B and NHBE cells were tested for NF-kB induced gene transcription using the Cignal Finder reporter system (SABiosciences). While BDE exposure caused a significant increase (p<0.001) in NF-kB mediated production of the transfected reporter luciferase gene, there was a decrease in this activity at 9% CO2 (p<0.05) in both BEAS-2B and NHBE cells compared to 5% CO2 (Fig. 7A-B) as seen by others (Takeshita et al., 2003,Cummins et al., 2010). Internal controls of the assay further indicated similar growth of both cell types under different CO2 incubation conditions.
Figure 7.
Elevated CO2 reduces the expression of NF-kB translocation in BEAS-2B and NHBE cells. (A) BEAS-2B and (B) NHBE cells were treated for 24 hr with media alone or 5% v/v barn dust and incubated at 5% or 9% CO2. Both cell types show a significant increase in NF-kB mediated luciferase expression with exposure to barn dust that is significantly reduced at 9% CO2 levels. Statistical significance * P<0.05, **** P<0.0001. (n=9)
Discussion
The elimination of CO2 from the body is a finely regulated system, and one that is very ancient in the development of aerobic multicellular organisms. Blockages of the airspace can quickly lead to elevation of CO2 with resulting hypercapnia in the blood (Simard et al., 1995). It is plausible that immunological sensing and response to CO2 elevation would modify innate immunity. Alveolar epithelial cells under hypercapnic conditions are subject to other changes as well, such as significantly impaired fluid re-absorption (Briva et al., 2007).
The airway epithelium is capable of producing a variety of cytokines and chemokines in response to innate immune ligands, and is instrumental in removal of debris, mucus, and organisms through cilliary action (Wanner et al., 1996). There is also evidence that they can act as antigen presenting cells as well (Salik et al., 1999). Therefore, if these cells are altered by changes to CO2, it may impact response to lung infections and other inhaled substances.
In past studies, TLR ligands, particularly Pam3CSK4 and LPS, can induce pro-inflammatory cytokine expression in BEAS-2B cells (Elson et al., 2007,Sha et al., 2004). These changes are shown to occur through the TLR pathway, resulting in a number of changes, including not only activation of NF-κB, triggering expression of a wide variety of immune function genes (Tian and Brasier, 2003), but also through improving sensitivity to these ligands via increases in TLR receptor expression (Vitseva et al., 2008). More complex immune stimuli, such as hog barn dusts, act through these same receptors and possibly through a host of others as well (Palmberg et al., 1998,Romberger et al., 2002). Hog barn dust is a complex stimulus with implications to farm worker health. It contains a complex mixture of bacterial, viral, and fungal proteins and molecules, amongst them peptidoglycan (TLR2 stimulus) and LPS (TLR4 stimulus). This is in addition to the particulate matter from hogs and feed. Most of the larger particulate matter is not present in these samples due to sterile filtering of samples, but smaller particles may have some effect (Li et al., 2003).
Our initial inflammatory cytokine work with IL-6 and IL-8 showed that there was a reduction in cytokine expression in Pam3CSK4 and LPS-stimulated cells starting at about 7% CO2 (Fig. 1). With BDE stimulation however there was an initial reduction in cytokine production at 7% followed by a sharp increase at 9% CO2 (Fig. 1G, 1H). While LPS (TLR4) and peptidoglycan (TLR2) ligands have been suggested as major immune stimulatory components of barn dust, whole barn dust may induce different responses to these individual ligands under elevated CO2. This could be due to a number of factors, some of which may alter LPS and peptidoglycan responses, and which may be susceptible to hypercapnic conditions. BDE + 9% CO2 increases in IL-6 and IL-8 production (P<0.05) were confirmed in NHBE cells. Results for IL-8 were further confirmed in BEAS-2B cells with a significant increase in IL-8 mRNA in cells when given BDE + 9% CO2 (p<0.05). IL-6 under these same conditions showed a rise in IL-6 mRNA expression over BDE alone, but the results were not significant.
A wide variety of chemokines were produced by these cells in response to the barn dust as measured by chemokine array (Fig. 3). Those found at the highest levels with some of the most pronounced changes due to CO2 were GRO, GRO-α (CXCL1), MCP-1 (CCL2), and MIP-1α (CCL3). Interestingly, these chemokines, along with IL-8, are all NF-κB regulated (Barnes and Karin, 1997) and were elevated at 9% CO2 compared to 5% CO2. However, our results clearly suggest a decrease in NF-kB activation in BEAS-2B and NHBE cells when given BDE + 9% CO2, a result that agrees with results found in pulmonary artery cells (Takeshita et al., 2003) and peripheral blood mononuclear cells (Cummins et al., 2010). We are not sure of the full implications of this result at present. This may suggest that some of the effects of hypercapnia on these cells may be at level of mRNA stability or translation instead of at mRNA transcription. When combined with our RT- PCR results showing an increase in IL-8 mRNA under hypercapnic conditions (Fig. 5B) in this NF-kB controlled gene, alterations in mRNA stability or increases in other transcription factors associated with IL-8 mRNA transcription seem likely (Fig. 7A-B). For example, p38 MAPK is known to play a role in IL-8 mRNA stability. Alternately, changes in other associated transcription factors, such as ERK or JNK (Hoffmann et al., 2002), may also be factors. While no changes were noted for several of these in THP-1 cells (Wang et al., 2010), the changes we note in the epithelial cells for indicators of NF-kB activity suggest differences in signaling between the two cell types in response to hypercapnia. RNA stability does not explain IL-6, however, as this mechanism has been shown not to play a role in hypercapnic changes in IL-6 (Wang et al., 2010). We note that similar to Wang et al., we see a decrease in IL-6 expression that is counter to what would be predicted from changes (or lack thereof) to NF-kB reporter gene activation or migration of subunits to the nucleus. This further demonstrates that changes to IL-6 as a result of hypercapnia are not driven by NF-kB, but rather by another transcriptional activator or inhibitor, as was proposed (Wang et al., 2010). More work needs to be done to identify this factor or factors.
There is some concern about the use of a dual-luciferase assay in NHBE cells given that these primary cells are less susceptible to transfection than cell lines. However, these cells are indeed capable of being transfected(Matsui et al., 1997,Maurisse et al., 2010). The key to this may be the use of shorter non-confluent cultures(Matsui et al., 1997) that are actively dividing, such as we and others (Wong et al., 2008,Han et al., 2005) have used with the dual-luciferase assay.
Further examination of the chemokine MCP-1 showed that while the pattern of its expression was similar to that of IL-6 and IL-8 in cells exposed to barn dust, the responses to Pam3CSK4 and LPS were quite different. Unlike IL-6 and IL-8, MCP-1 was increased with increasing hypercapnia in Pam3CSK4 and LPS treated cells (Fig.4B, 4C). MCP-1 showed a clear stepwise increase in Pam3CSK4 treated cells, starting at 7% CO2, whereas LPS, while recording greater end levels, showed no significant increases until 9% CO2. Of further interest, while media controls showed no detectible levels of MCP-1 at 5, 7, or 8% CO2, there was a significant increase at 9% (Fig. 5A; p<0.001). While this increase was significant, the increases in the treatment groups were larger and, as with Pam3CSK4, apparent at lower levels of CO2. We could also not find detectable levels of MCP-1 in our NHBE cells, so these changes may be specific to the cell line.
Because TLR receptor up-regulation due to TLR stimulation has been reported (Vitseva et al., 2008), we examined TLR receptor expression to see if this could account for the changes we observed. Surprisingly, no significant changes were seen for any receptor measured. This may be partly due to sample variation, but there was no clear indication of receptor expression being a significant reason for such changes in response to changing CO2 levels.
These results suggest several interesting features. First, airway epithelial cells respond to various immune stimuli differently in response to hypercapnia. Chemokine levels elicited by barn dust were all elevated in response to increased CO2 whereas with specific single TLR ligands (LPS and Pam3CSK4), there were decreases in IL-8 and increases in MCP-1 levels in response to elevated CO2 levels. Second, sensitivity to hypercapnia also appeared to be influenced by the stimulus in question. TLR ligands demonstrated cytokine increases beginning at 7%, whereas whole barn dust required 9% CO2 before eliciting an increased chemokine response.
While barn dust showed increases in all cytokines and chemokines tested, the results for LPS and Pam3CSK4 raise further questions. There may be an effect of hypercapnia on not just the intensity but also the character of inflammation. One possibility is the increase in MCP-1 could be a protective measure to decreasing inflammation as it has been shown to protect against lethal endotoxemia, increasing levels of IL-10 and reducing IL-12 (Zisman et al., 1997,Ramnath et al., 2008). MCP-1 is known to induce migration of T cells, mast cells, basophils, neutrophils, and monocytes (Chensue et al., 1996,Balamayooran et al., 2011,Leonard and Yoshimura, 1990); whereas, IL-8 is primarily involved in inducing migration of and activating neutrophils (Huber et al., 1991). Additionally, MCP-1 is known to play a role in macrophage activation (Jiang et al., 1992), but also TH2 cytokine production (Chensue et al., 1996,Gu et al., 2000). This is particularly interesting in the case of TLR stimulation, where the pattern of response is clearly associated with TH1-type responses (Takeda et al., 2003). If the effects of MCP-1 are anti-inflammatory, as Zisman et al. (1997) propose, it suggests hypercapnia in bronchial epithelial cells may have an anti-inflammatory effect with TLR ligand stimulation. Others, however, have shown that MCP-1 is pro-inflammatory and important in microbial clearance in disorders such as COPD (Blease et al., 2000,Balamayooran et al., 2011,Chung, 2001), leading to a less clear picture of inflammation due to hypercapnia in BEAS-2B cells. In the case of stimulation with barn dust under hypercapnic conditions, increasing IL-8 and MCP-1 may be a response to strong pro-inflammatory signals from many sources in this complex stimulus.
Future studies will need to assess the parameters of hypercapnic increases in MCP-1. For example, MCP-1 is induced at maximal levels fairly early in a response, at about 2 hr, with a decline to baseline at around 48 hr in lungs and serum from mice challenged with LPS (Zisman et al., 1997). Therefore, the increases seen at 24 hr could be a kinetics change. Given that MCP-1 can be induced by hypercapnia, some of the changes seen in other cytokines and chemokines may attributable to the actions of MCP-1 itself.
Alterations to the NF-κB pathway are common in activation of innate immune responses (Takeshita et al., 2003). As hypercapnia can alter these responses it is reasonable, and indeed shown that hypercapnia can also affect NF-kB translocation and signaling, although the type of alteration seems to depend on the system used (Abolhassani et al., 2009,Briva et al., 2007,Wang et al., 2010,Cummins et al., 2010). Our results in testing for NF-kB activation indicate a reduction in such activity with response to BDE plus elevated CO2 (Fig. 7A-B). This may also be a response that is specific to the cell type or stimulus used. Barn dust is also a complex stimuli, and responses may be different compared to some more simple stimuli.
It is clear that determinations of the effects of hypercapnia must be carefully considered. A given ligand or infection may need to be tested at several levels of CO2, measuring several cytokines/chemokines to be sure that effects are not being missed. Determination of pro- or anti-inflammatory effects of hypercapnia may need to be determined by studies of cellular influx into the lung combined with cytokine and chemokine assays. This however is hard to do in humans, requiring a reliance on cell cultures. We show here what appears to be generally an anti-inflammatory response of hypercapnia in a bronchial epithelial cell line to LPS and PAM3CSK, but a pro-inflammatory response to organic barn dust. The anti-inflammatory effects seen with common bacterial ligands may help to explain some of the results seen in earlier ventilator studies (Laffey and Kavanagh, 1999) and would be more pertinent to most cases of ARDS seen. This work however also shows that more complex inflammatory stimuli such as barn dusts can induce responses that can differ greatly from single immune stimulatory ligands and respond differently to hypercapnia.
Supplementary Material
Highlights.
Airway epithelial cell immune responses to barn dust respond differently to hypercapnic conditions than individual TLR ligands.
We show here an anti-inflammatory response of hypercapnia in a bronchial epithelial cell line to LPS and PAM3CSK, but a pro-inflammatory response to organic barn dust.
In the case of stimulation with barn dust under hypercapnic conditions, increasing IL-8 and MCP-1 may be a response to strong pro-inflammatory signals from many sources in this complex stimulus.
Acknowledgements
We gratefully acknowledge Lisa Chudomelka for helping to edit this paper.
Funding information: Study supported by grants from the National Institute of Alcohol Abuse and Alcoholism (R01AA017993 to TAW), and National Institute of Occupational Safety Health (R01OH008539-01 to DJR and 1U54OH010162-01 to TAW). This work was supported in part by the Central States Center for Agricultural Safety and Health (CS-CASH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abolhassani M, Guais A, Chaumet-Riffaud P, Sasco AJ, Schwartz L. Carbon dioxide inhalation causes pulmonary inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L657–65. doi: 10.1152/ajplung.90460.2008. [DOI] [PubMed] [Google Scholar]
- Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect. Immun. 2011;79:2567–2577. doi: 10.1128/IAI.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-κB - A pivotal transcription factor in chronic inflammatory diseases. New England Journal of Medicine. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Begin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1991;143:905–912. doi: 10.1164/ajrccm/143.5_Pt_1.905. [DOI] [PubMed] [Google Scholar]
- Blease K, Mehrad B, Standiford TJ, Lukacs NW, Gosling J, Boring L, Charo IF, Kunkel SL, Hogaboam CM. Enhanced pulmonary allergic responses to Aspergillus in CCR2(−/−) mice. Journal of Immunology. 2000;165:2603–2611. doi: 10.4049/jimmunol.165.5.2603. [DOI] [PubMed] [Google Scholar]
- Briva A, Vadasz I, Lecuona E, Welch LC, Chen J, Dada LA, Trejo HE, Dumasius V, Azzam ZS, Myrianthefs PM, Batlle D, Gruenbaum Y, Sznajder JI. High CO2 levels impair alveolar epithelial function independently of pH. PLoS One. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue SW, Warmington KS, Ruth JH, Sanghi PS, Lincoln P, Kunkel SL. Role of Monocyte Chemoattractant Protein-1 (MCP-1) in Th1 (Mycobacterial) and Th2 (Schistosomal) Antigen-Induced Granuloma Formation: Relationship to Local Inflammation, Th Cell Expression, and IL-12 Production. Journal of Immunology. 1996;157:4602–4608. [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. European Respiratory Journal, Supplement. 2001;18:50s–59s. [PubMed] [Google Scholar]
- Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, Slattery C, Leonard MO, McLoughlin P, Taylor CT. NF-κB links CO2 sensing to innate immunity and inflammation in mammalian cells. Journal of Immunology. 2010;185:4439–4445. doi: 10.4049/jimmunol.1000701. [DOI] [PubMed] [Google Scholar]
- Curley G, Contreras MM, Nichol AD, Higgins BD, Laffey JG. Hypercapnia and acidosis in sepsis: a double-edged sword? Anesthesiology. 2010;112:462–472. doi: 10.1097/ALN.0b013e3181ca361f. [DOI] [PubMed] [Google Scholar]
- Dong H, Kang G, Zhu Z, Tao X, Chen Y, Xin H, Harmon JD. Ammonia, methane, and carbon dioxide concentrations and emissions of a hoop grower-finisher swine barn. Transactions of the ASABE. 2009;52:1741–1747. [Google Scholar]
- Dong H, Zhu Z, Shang B, Kang G, Zhu H, Xin H. Emissions of greenhouse gases from a typical Chinese swine farrowing barn. Transactions of the ASABE. 2007;50:1037–1044. [Google Scholar]
- Donham KJ, Cumro D, Reynolds SJ, Merchant JA. Dose-response relationships between occupational aerosol exposures and cross-shift declines of lung function in poultry workers: recommendations for exposure limits. J. Occup. Environ. Med. 2000;42:260–269. doi: 10.1097/00043764-200003000-00006. [DOI] [PubMed] [Google Scholar]
- Dosman JA, Fukushima Y, Senthilselvan A, Kirychuk SP, Lawson JA, Pahwa P, Cormier Y, Hurst T, Barber EM, Rhodes CS. Respiratory response to endotoxin and dust predicts evidence of inflammatory response in volunteers in a swine barn. Am. J. Ind. Med. 2006;49:761–766. doi: 10.1002/ajim.20339. [DOI] [PubMed] [Google Scholar]
- Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J. Contribution of toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 2007;109:1574–1583. doi: 10.1182/blood-2006-06-032961. [DOI] [PubMed] [Google Scholar]
- Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, Beitel GJ, Hauser AR, Sznajder JI, Sporn PH. Hypercapnia Impairs Lung Neutrophil Function and Increases Mortality in Murine Pseudomonas Pneumonia. Am. J. Respir. Cell Mol. Biol. 2013 doi: 10.1165/rcmb.2012-0487OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of T(H)2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Han W, Pentecost BT, Pietropaolo RL, Fasco MJ, Spivack SD. Estrogen receptor alpha increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1, in normal human bronchial epithelial cells. Mol. Carcinog. 2005;44:202–211. doi: 10.1002/mc.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Huber AR, Kunkel SL, Todd III RF, Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. Journal of Immunology. 1992;148:2423–2428. [PubMed] [Google Scholar]
- Just N, Duchaine C, Singh B. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J. Occup. Med. Toxicol. 2009;4:13–6673-4-13. doi: 10.1186/1745-6673-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirychuk SP, Dosman JA, Reynolds SJ, Willson P, Senthilselvan A, Feddes JJ, Classen HL, Guenter W. Total dust and endotoxin in poultry operations: comparison between cage and floor housing and respiratory effects in workers. J. Occup. Environ. Med. 2006;48:741–748. doi: 10.1097/01.jom.0000216215.39521.3c. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill--too little of a good thing? Lancet. 1999;354:1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol. Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsui H, Johnson LG, Randell SH, Boucher RC. Loss of binding and entry of liposome-DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J. Biol. Chem. 1997;272:1117–1126. doi: 10.1074/jbc.272.2.1117. [DOI] [PubMed] [Google Scholar]
- Maurisse R, De Semir D, Emamekhoo H, Bedayat B, Abdolmohammadi A, Parsi H, Gruenert DC. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnol. 2010;10:9–6750-10-9. doi: 10.1186/1472-6750-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol AD, O'Cronin DF, Howell K, Naughton F, O'Brien S, Boylan J, O'Connor C, O'Toole D, Laffey JG, McLoughlin P. Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit. Care Med. 2009;37:2953–2961. doi: 10.1097/CCM.0b013e3181b028ce. [DOI] [PubMed] [Google Scholar]
- Norman V. An overview of the vapor phase, semivolatile and nonvolatile components of cigarette smoke. Recent Advances in Tobacco Science. 1977;3:28–51. [Google Scholar]
- O'Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O'Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax. 2009;64:976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- Palmberg L, Larsson B, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax. 1998;53:260–264. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Curr. Opin. Allergy Clin. Immunol. 2012;12:126–132. doi: 10.1097/ACI.0b013e3283511d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Von Essen SG, Hervert J, Parks C, Mathisen T, Romberger DJ. Repeat organic dust exposure-induced monocyte inflammation is associated with protein kinase C activity. J. Allergy Clin. Immunol. 2007;120:366–373. doi: 10.1016/j.jaci.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Rai S, Engelberts D, Laffey JG, Frevert C, Kajikawa O, Martin TR, Post M, Kavanagh BP. Therapeutic hypercapnia is not protective in the in vivo surfactant-depleted rabbit lung. Pediatr. Res. 2004;55:42–49. doi: 10.1203/01.PDR.0000098502.72182.55. [DOI] [PubMed] [Google Scholar]
- Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int. Immunopharmacol. 2008;8:810–818. doi: 10.1016/j.intimp.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J. Appl. Physiol. 2002;93:289–296. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- Salik E, Tyorkin M, Mohan S, George I, Becker K, Oei E, Kalb T, Sperber K. Antigen trafficking and accessory cell function in respiratory epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 1999;21:365–379. doi: 10.1165/ajrcmb.21.3.3529. [DOI] [PubMed] [Google Scholar]
- Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. American Journal of Respiratory Cell and Molecular Biology. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- Simard A, Maltais F, LeBlane P. Functional outcome of patients with chronic obstructive pulmonary disease and exercise hypercapnia. European Respiratory Journal. 1995;8:1339–1344. doi: 10.1183/09031936.95.08081339. [DOI] [PubMed] [Google Scholar]
- Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2002;166:403–408. doi: 10.1164/rccm.200112-117OC. [DOI] [PubMed] [Google Scholar]
- Stinn JP, Xin H, Shepherd T, Li H, Burns R. Ammonia and greenhouse gas emissions of a swine breeding-gestation- farrowing facility in the midwestern USA. ASABE - 9th International Livestock Environment Symposium 2012. 2012:359–366. ILES 2012. [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Suzuki Y, Nishio K, Takeuchi O, Toda K, Kudo H, Miyao N, Ishii M, Sato N, Naoki K, Aoki T, Suzuki K, Hiraoka R, Yamaguchi K. Hypercapnic acidosis attenuates endotoxin-induced nuclear factor-[kappa]B activation. Am. J. Respir. Cell Mol. Biol. 2003;29:124–132. doi: 10.1165/rcmb.2002-0126OC. [DOI] [PubMed] [Google Scholar]
- Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog. Horm. Res. 2003;58:95–130. doi: 10.1210/rp.58.1.95. [DOI] [PubMed] [Google Scholar]
- Vaporidi K, Tsatsanis C, Georgopoulos D, Tsichlis PN. Effects of hypoxia and hypercapnia on surfactant protein expression proliferation and apoptosis in A549 alveolar epithelial cells. Life Sci. 2005;78:284–293. doi: 10.1016/j.lfs.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Vitseva OI, Tanriverdi K, Tchkonia TT, Kirkland JL, McDonnell ME, Apovian CM, Freedman J, Gokce N. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadasz I, Chandel NS, Sznajder JI. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011;286:37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Gates KL, Trejo H, Favoreto S, Jr, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PH. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24:2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. American Journal of Respiratory and Critical Care Medicine. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Wong SH, Shih RS, Schoene NW, Lei KY. Zinc-induced G2/M blockage is p53 and p21 dependent in normal human bronchial epithelial cells. Am. J. Physiol. Cell. Physiol. 2008;294:C1342–9. doi: 10.1152/ajpcell.00061.2008. [DOI] [PubMed] [Google Scholar]
- Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, Standiford TJ. MCP-1 protects mice in lethal endotoxemia. J. Clin. Invest. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.