Abstract
The gene SLC4A5 encodes the Na+-HCO3− co-transporter electrogenic 2 (NBCe2), which is located in the distal nephron. Genetically deleting NBCe2 (KO) causes Na+-retention and hypertension, a phenotype that is diminished with alkali loading. We performed experiments with acid-loaded mice and determined whether over-active epithelial Na+ channels (ENaC) or the Na+-Cl− co-transporter (NCC) causes the Na+ retention and hypertension in KO. In untreated mice, the mean arterial pressure (MAP) was higher in KO, compared with wild type (WT); however, treatment with amiloride, a blocker of ENaC, abolished this difference. In contrast, hydrochlorothiazide (HCTZ), an inhibitor of NCC, decreased MAP in WT, but not KO. Western blots showed that quantity of plasmalemmal full-length ENaC-α was significant higher in KO than in WT. Amiloride treatment caused a 2-fold greater increase in Na+ excretion in KO, compared with WT. In KO, but not WT, amiloride treatment decreased plasma [Na+] and urinary K+ excretion, but increased hematocrit and plasma [K+] significantly. Micropuncture with microelectrodes showed that the [K+] was significantly higher and the transepithelial potential (Vte) was significantly lower in the late distal tubule (LDT) of the KO compared with WT. The reduced Vte in KO was amiloride-sensitive and therefore revealed an upregulation of electrogenic ENaC-mediated Na+ reabsorption in this segment. These results show that, in the absence of NBCe2 in the LDT, acid-loaded mice exhibit disinhibition of ENaC-mediated Na+ reabsorption, which results in Na+ retention, K+ wasting, and hypertension.
Keywords: hypertension, distal nephron, NBCe2, ENaC, micropuncture
INTRODUCTION
The protein NBCe2 (electrogenic Na+-HCO3− co-transporter 2), encoded by the SLC4A5 gene, has been identified in choroid plexus (1), liver (2) and kidney (3), where it is expressed in the distal nephron (4). Functional studies indicate that NBCe2 mediates Na+-HCO3− influx with a stoichiometry of 1:2 or 1:3 and is involved in intracellular pH regulation (1;5;6).
Polymorphisms in the SLC4A5 gene are associated with hypertension in humans (7–9). A previous study suggested that the hypertension exhibited by an NBCe2 knockout (KO) is linked to renal metabolic acidosis (4). The detailed pathogenesis of the KO hypertension was not established. However, the investigators suspected an up-regulation of one of the recently identified Na+ reabsorbing transporters in the distal nephron (4).
A primary defect in Na+ reabsorption by NBCe2-KO could result from enhanced Na+ reabsorption by the epithelial Na+ channels (ENaC), the Na+-Cl− co-transporter (NCC), or the recently described Na+-dependent Cl−/HCO3− exchanger (NDCBE) (10). We distinguished ENaC-mediated Na+ retention from Na+-co-transporters by its specific sensitivity to amiloride and its association with K+ wasting. We distinguished NCC and NDCBE from ENaC by their sensitivity to hydrochlorothiazide (HCTZ). In addition, we performed in vivo micropuncture with microelectrodes to distinguish between the electrogenic effect of ENaC activation and an electroneutral effect of increased NCC and NDCBE-mediated Na+ reabsorption in the distal nephron.
METHODS
Animal Studies
Wild type (WT) mice (C57Bl/6) were purchased from Charles River (Wilmington). NBCe2 knockout (KO) mice (129) were generated as described previously (4). The KO were backcrossed to C57Bl/6 for at least 6 generations using marker assisted speed congenics (11). All mice were maintained in accordance with the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. For acid loading experiments, mice (12–20 week-old) were fed an acid diet (1.5% NH4Cl, Harlan Laboratories) for 7 days. Mean arterial pressure (MAP) was then measured using CODA-6 tail-cuff system (Kent Scientific) as described before (12;13). For diuretic experiments, amiloride and HCTZ were administered in the drinking water (25 mg/L) for 4 days after acid loading. For metabolic cage experiments, mice were acclimated for 24 hours and then urine was collected for 12 hours as described previously (14). A single dose of vehicle (PEG) or amiloride (5 mg/kg) was injected intraperitoneally. Arterial blood electrolyte values were analyzed with the MN: 300 i-STAT system (Abbott Point of Care), while the hematocrit was measured by capillary tube. Urine [Na+] and [K+] were analyzed with a flame photometer (Jenway Clinical PFP7). The urinary amiloride and HCTZ concentrations were measured by HPLC, and the luminal [amiloride/HCTZ] in the terminal cortical collecting duct (CCD) was calculated as: urine [amiloride/HCTZ] × plasma osmolality / urine osmolality, as described in a previous study from our laboratory (14).
Plasma membrane protein isolation and Western blotting
The plasma membrane fraction of the kidney cortex was isolated using the Minute™ Plasma Membrane Protein Isolation Kit (Invent Biotechnologies Inc.) (15). Western blotting was performed as described previously by this laboratory (15). The primary antibodies were anti-ENaC-α and NCC (rabbit polyclonal, diluted 1:500, Stressmarq), and anti-cadherin (goat polyclonal, diluted 1:500, Santa Cruz), with goat anti-rabbit IgG, or donkey anti-goat IgG conjugated HRP (diluted 1:10,000, Santa Cruz). Expression of proteins was quantified by densitometry using Quantity One (Bio-Rad).
Micropuncture, Vte and tubular [K+] measurements
Micropuncture experiments of mice kidney tubules was performed as described previously (16;17) (Supplementary).
Statistical analyses
Data shown in figures represent mean ± SEM. Significant differences between each group were determined by Student’s t-test or ANOVA analyses plus Student-Newman-Keuls test (P < 0.05 considered significant).
RESULTS
Origin of hypertension in NBCe2-KO mice
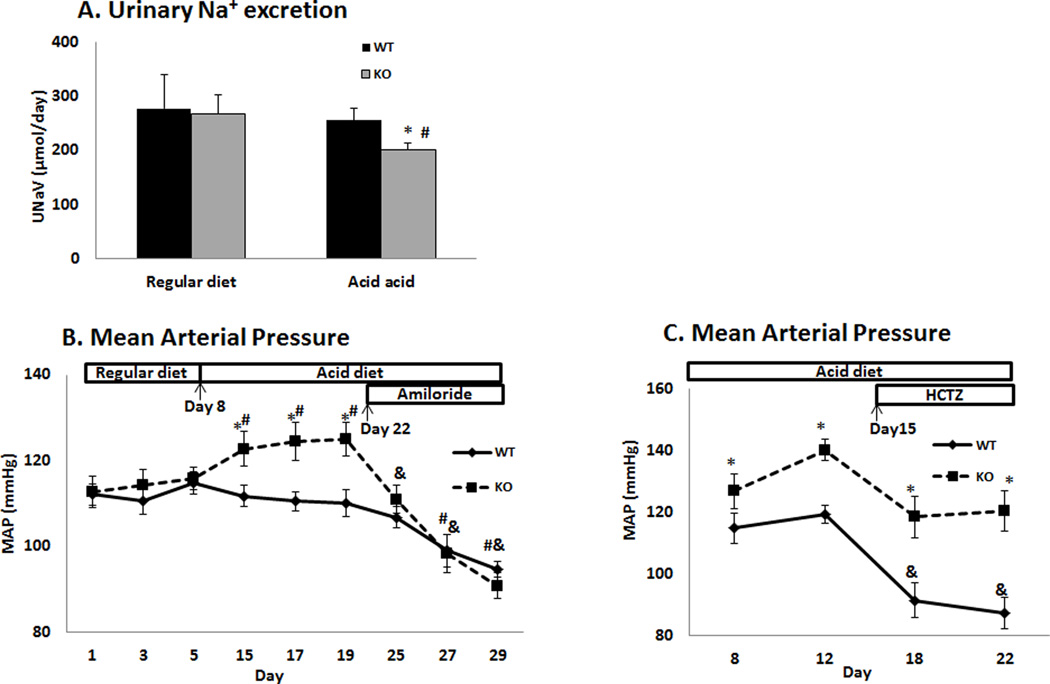
As shown in figure 1A, the urinary Na+ excretion rate (UNaV, µmol/day) was similar between WT (277.1 ± 62.4, n = 4) and KO (267.9 ± 34.0, n = 4) when they were on regular diet, but it was significantly decreased in the KO after an acid diet for 3 days (201.8 ± 12.4, n = 9), as compared to WT (255.6 ± 22.9, n = 8). No significant difference in the diet/water intake was found between each group. This indicates Na+ retention in the KO after acid loading.
Figure 1. Urinary Na+ excretion and blood pressure in WT and KO.
(A) Urinary Na+ excretion rate (UNaV) in WT and KO on regular diet and after 3 days of acid diet. (B) Tracing of MAP in WT and KO on regular diet, acid diet and after treatment with amiloride. Acid diet was started on Day 8, and amiloride was administered on Day 22. (C) Tracing of MAP in WT and KO before and after treatment with HCTZ. HCTZ was administered on Day 15. (* P < 0.05 vs WT, # P < 0.05 vs regular diet, & P < 0.05 vs acid diet).
Blood pressure was then measured in WT and KO. As shown in figure1B and 1C, the MAP (mmHg) was similar between WT (n = 9) and KO on regular diet (n = 8). However, it was significantly increased in the KO as compared to WT after acid diet for 7 days. This difference in MAP was eliminated by treatment with amiloride. However, treatment with HCTZ decreased the MAP in the WT, but not KO (n = 5/5), and the difference in MAP remained. These results show that the increased activity of ENaC, but not NCC or NDCBE, contributes to the hypertension caused by NBCe2 deficiency.
Using HPLC, the calculated luminal concentrations of amiloride and HCTZ in the terminal CCD were 5.6 ± 1.0 µM (n = 4) and 241.0 ± 9.6 µM (n = 4), respectively, which were high enough to inhibit ENaC and NCC/NDCBE (10). However, this concentration of luminal amiloride would not inhibit the NHE, which has a Ki of more than 40 µM (18).
ENaC activity and K+ excretion in NBCe2-KO
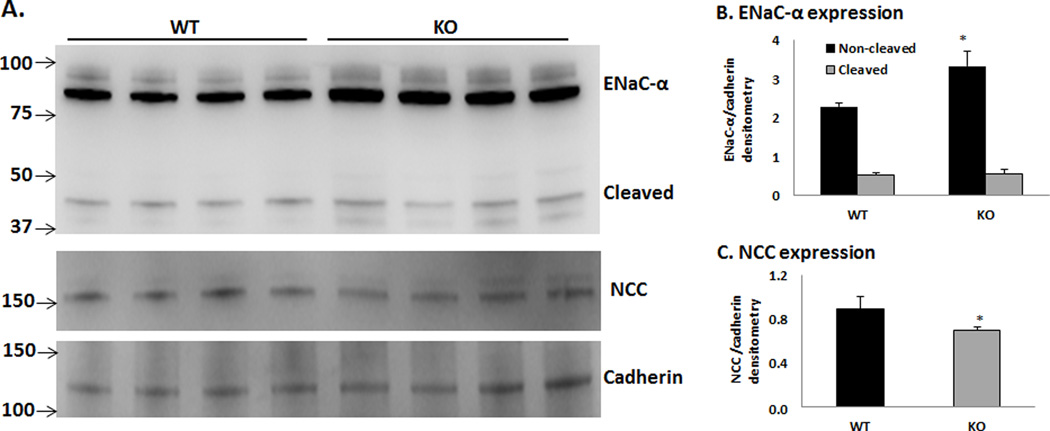
Western blot analysis was performed to determine the protein expressions of the ENaC-α and NCC in the plasma membranes of the kidney cortex of WT vs KO. As shown in figure 2, the non-cleaved ENaC-α protein was significantly greater in the KO compared to WT; however, the level of cleaved ENaC-α expression was not different between each group. These results indicate that the ENaC is activated in the KO while the NCC is inhibited as a result of increased ENaC activity (19,20).
Figure 2. Expressions of ENaC-α and NCC in renal plasma membranes of WT and KO on acid diet.
N = 4 in each group; *P < 0.05 vs WT.
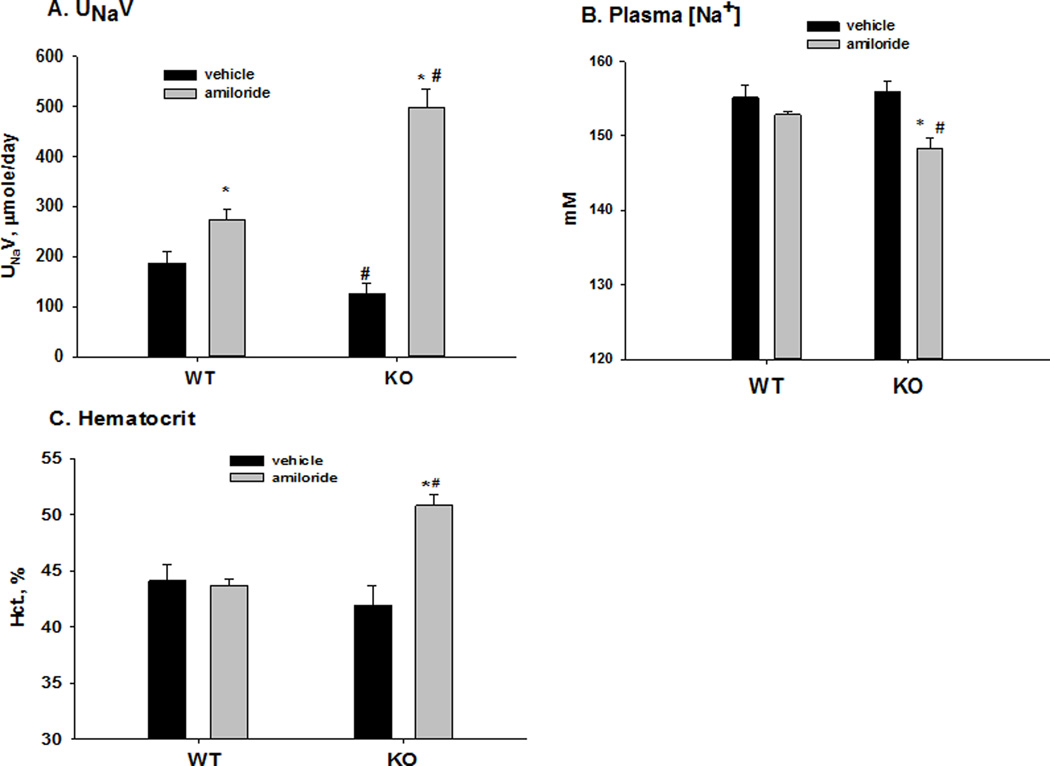
Metabolic cage experiments were then used to examine the function of ENaC in WT and KO. The food intake was similar between WT and KO (0.44 ± 0.15 vs 0.56 ± 0.15, n= 5/7, P = 0.601), as well as the water intake (1.04 ± 0.31 vs 1.33 ± 0.17, n= 5/7, P = 0.399). Figure 3 shows the amiloride-sensitive rate of urinary Na+ excretion, plasma [Na+], and hematocrits, indications of the amount of ENaC-mediated Na+ and volume retention in WT and KO mice. As shown in 3A, the urinary Na+ excretion (µmol/day) in WT with vehicle was 186.7 ± 23.7 (n = 5) and was significantly greater with amiloride treatment (274.2 ± 19.3; n = 11). In KO, the value for Na+ excretion (µmol/day) with vehicle was 126.5 ± 20.8 (n = 5), which was significantly less than WT. On treatment with amiloride, the value for Na+ excretion increased to 499.2 ± 35.9 (n = 8), which was significantly greater than vehicle WT, demonstrating a large increase in amiloride-sensitive Na+ reabsorption in the KO. As shown in 3B, amiloride treatment significantly decreased the plasma [Na+] in the KO (vehicle: 156.0 ± 1.4; amiloride: 148.3 ± 1.4; n = 7/6) but not WT (vehicle: 155.1 ± 1.7; amiloride: 152.8 ± 0.4; n = 7/6). The hematocrit, an indicator of plasma volume, was increased in the KO (vehicle: 42.0 ± 1.7; amiloride: 50.3 ± 1.0; n = 11/8) but not WT (vehicle: 44.1 ± 1.5; amiloride: 45.7 ± 1.5; n = 5/5) with amiloride treatment. These results demonstrate considerable ENaC-mediated Na+ reabsorption and volume retention in the KO.
Figure 3. Effects of amiloride on urinary Na+ excretion (A), plasma [Na+] (B), and hematocrit (C) in WT and KO with acid loading.
* P < 0.05 vs vehicle; # P <0.05 vs WT.
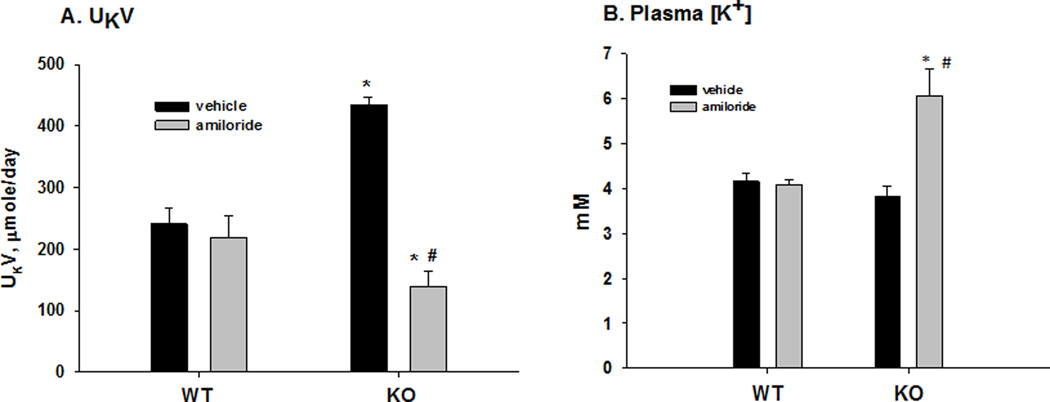
As shown in figure 4A, the rate of urinary K+ excretion (µmol/day) was not significantly different when WT treated with vehicle (240.6 ± 25.5; n = 6) was compared with amiloride treatment (219.0 ± 35.8; n = 11). However, for KO, the value of K+ excretion with vehicle (435.2 ± 11.6; n = 5) was significantly greater than that of amiloride-treated mice (140.0 ± 23.7; n = 8). Thus, KO exhibited a large amiloride-sensitive K+ sparing effect that was not present in WT. The effects of amiloride on the plasma [K+] values for WT and KO are shown in figure 4B. As shown, the plasma [K+] was increased with amiloride treatment in the KO (vehicle: 3.8 ± 0.1; amiloride: 6.1 ± 0.6; n = 7/6) but not the WT (vehicle: 4.2 ± 0.2; amiloride: 4.1 ± 0.2; n = 7/6).
Figure 4. Effects of amiloride on urinary K+ excretion (A), and plasma [K+] (B) in WT and KO with acid loading.
* P < 0.05 vs vehicle; # P <0.05 vs WT.
Micropuncture assessment of ENaC activity
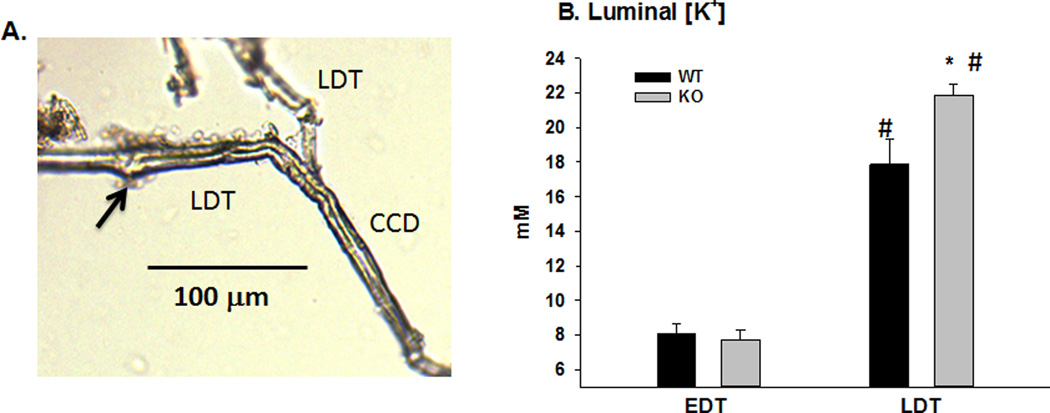
Micropuncture was used to further investigate the ENaC activity in the distal nephron of KO. Figures 5 and 6 show the results of in vivo conventional and K+-selective microelectrode measurements to determine the site of ENaC-mediated Na+ reabsorption and K+ wasting of WT and KO. The early distal tubule (EDT) was localized as the first site of appearance of fast green dye after its disappearance into the thick ascending limb. The late distal tubule (LDT) was the last site of appearance of fast green before descending below the surface and into the collecting ducts. Figure 5A is a photograph of a latex-filled micropunctured LDT, showing the site of micropuncture and its extension into the CCD. The average length for LDT measurements, from site of micro-puncture to entry into CCD, was 158.6 ± 18.0 µm (n = 7). This distance places the LDT measurements at the distal convoluted tubule (DCT2) and connecting tubule (CNT) transition as previously described (21).
Figure 5. Microelectrode localization and measurements of luminal [K+] in distal tubules.
A. Photograph of latex cast of late distal tubule (LDT) showing the distance from the site of microelectrode puncture (arrow) to the cortical collecting duct (CCD). B. Bar plots illustrating the luminal [K+] in the early (EDT) and late distal tubules (LDT). *P < 0.05 vs WT; # P < 0.05 vs EDT.
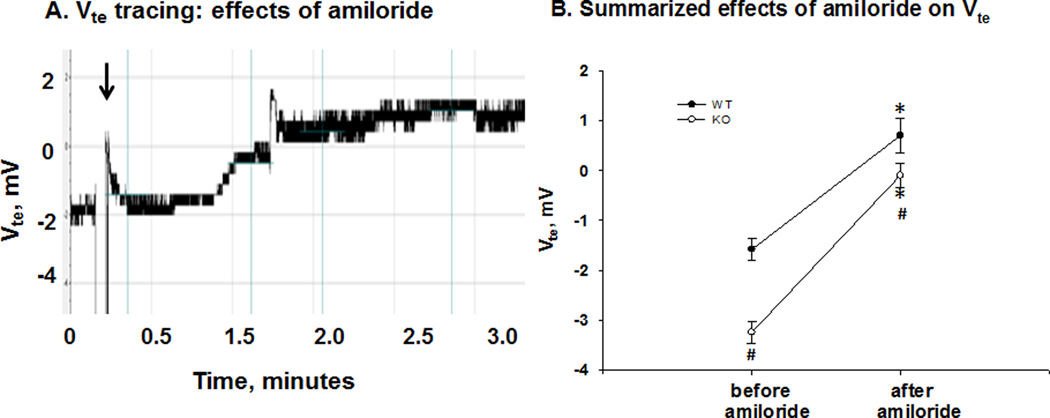
Figure 6. Effects of amiloride on Vte in the late distal tubule.
A. Tracing of Vte in late distal tubule of WT, illustrating an increase in stable Vte from 0 to 1.5 mV approximately 90 seconds after amiloride treatment (IP: arrow). B. Summary scatter plots of Vte (means ± SEM) of WT and KO before and after amiloride treatment. Amiloride increased the Vte more in the KO as compared with WT. (*P < 0.05 vs before amiloride; # P < 0.05 vs WT.)
As shown in figure 5B, using K+-selective microelectrodes, we found the [K+] in the EDT was not different in KO compared with WT; however, the [K+] was significantly higher in the LDT of KO mice, compared with WT (WT: 17.9 ± 1.5 vs KO: 21.9 ± 0.6 mM, n = 5/5).
Figure 6 shows the Vte in the LDT for WT and KO before and after amiloride injection. As shown by the Vte recording of LDT from WT in figure 5A, the luminal Vte was significantly increased to a higher stable value within two minutes after amiloride administration. As shown by the summary scatter plots of figure 6B, the late distal Vte (mV) of WT was −1.6 ± 0.2 (n = 6) before amiloride and 0.7 ± 0.4 (n = 6) after amiloride addition. The Vte (mV) of KO was −3.2 ± 0.2 (n = 7) before amiloride and significantly increased to −0.1 ± 0.2 (n = 7) with amiloride addition. Amiloride treatment caused a significantly greater increase in Vte of KO (ΔVte = 3.1 ± 0.1; n =7), compared with WT (ΔVte = 2.3 ± 0.2; n = 6). We are uncertain why the Vte was significantly greater in WT after amiloride treatment; however, the Vtes were not significantly different from zero after amiloride treatment, indicating little H+ secretion in WT and KO in this segment. Nevertheless, the greater amiloride-sensitive Vte in KO indicates increased ENaC activity in the LDT.
DISCUSSION
Our study reports hypertension in acid-loaded NBCe2-KO, associated with increased ENaC activity in the distal nephron. Our evidence included increased full-length ENaC-α expression in the renal plasma membrane, increased amiloride-sensitive Na+ reabsorption and K+ secretion, and increased [K+] and decreased Vte in the lumen of LDT. Treatment with amiloride decreased MAP more in the KO compared to WT. On the other hand, HCTZ decreased MAP in WT but not KO. The increases in plasma [Na+] and hematocrit of KO with amiloride treatment were consistent with excessive ENaC-mediated Na+ reabsorption with volume retention.
ENaC-mediated fluid retention and hypertension in NBCe2-KO
A previous study reported that the increased blood pressure in KO on a mixed SV129/C57 background was linked to renal metabolic acidosis. Moreover, alkali loading eliminated the difference in BP between WT and KO (4). In the present study, KO on a pure C57 background did not exhibit significantly enhanced MAP when on a regular diet. However, when given an acidic load, we found clear phenotypic differences between KO and WT with respect to fluid retention and hypertension. The acidic load may be more translational to the human Western diet, with its higher protein content that generates acid for renal excretion. However, a standard rodent chow provides a large dietary alkali load (22).
We discerned the Na+ reabsorbing pathway in the distal nephron with HCTZ, and amiloride. Our experiments showed that amiloride, but not HCTZ, ablated the hypertension and the Na+ retention of KO, with a large increase in Na+ excretion. In KO, amiloride also caused a large increase in the hematocrit, used as an indicator of plasma volume (13). The large increase in hematocrit with acute amiloride treatment indicated a large amount of volume retention associated with ENaC-mediated Na+ reabsorption.
The significantly reduced rate of K+ excretion and elevation in plasma [K+] in KO with amiloride treatment is additional evidence that enhanced ENaC activity is responsible for Na+ retention. The primary driving force for K+ secretion is the electronegative lumen potential generated by ENaC-mediated Na+ reabsorption.
One limitation of our current study is that we used tail-cuff to measure the MAP. Although we trained the mice to limit sympathetic stimulation, future studies using telemetry could yield more accurate information about the changes in BP after diuretic therapy (23).
Micropuncture experiments
Micropuncture with microelectrodes was used to obtain more direct evidence that ENaC, an electrogenic transporter, rather than an electroneutral Na+ co-transporter, was responsible for the Na+ retention and hypertension of KO. The [K+] in the LDT of WT of approximately 17 mM is similar to that of a previous study (24). The amiloride-sensitive Vte in KO was significantly greater than WT, consistent with enhanced ENaC-mediated Na+ reabsorption compared with WT. The greater [K+] of 23 mM in KO, compared with WT, demonstrates that the site of K+ wasting is evident in the CNT/DCT2 where ENaC is active.
Mechanism of enhanced ENaC activity in KO
The aldosterone signaling cascade, involving SGK-1 regulation of ENaC expression via inhibition of Nedd4-2, is well established. However, the plasma [aldosterone] is lower in KO, compared with WT, as shown previously (4). Thus, the activation of ENaC and K+ wasting in KO is not a result of hyperaldosteronism secondary to a failure of NBCe2-mediated Na+ reabsorption, but rather a primary defect of ENaC-mediated Na+ reabsorbing principal cells (PC). This effect is consistent with a role for NBCe2 to inhibit ENaC-mediated Na+ reabsorption when animals are on an acid diet.
The detailed localization of NBCe2 is not well-established. We propose that it may be a basolateral transporter like NBCn1 of the thick ascending limb (25), where it buffers the cell during acidosis. In the basolateral membrane, NBCe2 would have more access to high plasma HCO3− of 20–25 mM that would help drive the HCO3− into the cell against the cellular negative potential. However, the HCO3− concentration of the distal tubule lumen is very low with acidifying conditions. The acidosis causes H+ entry into the PC of the distal nephron, causing the activation and up-regulation of NBCe2 in order to buffer the cell and increase pHi. Increasing the activity of NBCe2 will effectively increase intracellular [Na+] and hyperpolarize the cell at the same time. The increased intracellular [Na+] will prevent ENaC-mediated Na+ reabsorption by negative feedback (26) and the hyperpolarization decreases the driving force for K+ secretion. The reduction of Na+ reabsorption via ENaC is associated with a reduction in Cl− reabsorption in exchange for HCO3− secretion via pendrin in β-intercalated cells, an effect that would exacerbate the acidosis. Therefore, the role of the NBCe2 in the PC will prevent HCO3− secretion and promote H+ secretion.
PERSPECTIVES
Single nucleotide polymorphisms (SNPs) in the gene SLC4A5/NBCe2 are associated with hypertension in humans. We used the NBCe2-KO mice to investigate the potential mechanism. Although the KO mouse model is different from the SNPs of SLC4A5/NBCe2 in the human condition, it provides important information on the NBCe2 function in vivo. Our study shows that increased activity of ENaC, but not NCC, in the LDT plays an important role in the pathogenesis of hypertension in the KO mice. Thus, amiloride is better than thiazide for the treatment of hypertension in KO.
Gain-of-function mutations in ENaC cause hypertension in humans, mice (27–30) and other animal models (31–33), illustrating the importance of ENaC in the regulation of blood pressure (34). It is important to identify hypertensive patients with SLC4A5 SNPs, and test the ENaC function/amiloride effect on the BP of these patients.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New
The pathogenesis of hypertension associated with NBCe2 deficiency is due to ENaC activation.
What Is Relevant
Understanding the pathogenesis of hypertension caused by NBCe2 deficiency and development of potential treatment for this disorder are critical as SLC4A5/NBCe2 polymorphisms are strongly associated with hypertension in humans.
Summary
An increase in ENaC- but not NCC-mediated Na+ reabsorption in the distal nephron plays an important role in hypertension caused by NBCe2 deficiency in mice. Amiloride is more effective than thiazide for the treatment of hypertension caused by SLC4A5/NBCe2 mutations.
ACKNOWLEDGMENTS
We thank Dr. Volker Vallon from the UC San Diego and Dr. Tong Wang from Yale University for the advice in the micropuncture experiments.
SOURCES OF FUNDING
This work was funded by National Institutes of Diabetes and Digestive and Kidney Diseases Grants RO1 DK071014 and RO1 DK73070 (SCS), and a fellowship (#11PRE7530018) from the American Heart Association MWA Affiliate (RJC).
Footnotes
DISCLOSURE
None.
Reference List
- 1.Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene) J Biol Chem. 2011;286:32563–32574. doi: 10.1074/jbc.M111.249961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abuladze N, Pushkin A, Tatishchev S, Newman D, Sassani P, Kurtz I. Expression and localization of rat NBC4c in liver and renal uroepithelium. Am J Physiol Cell Physiol. 2004;287:C781–C789. doi: 10.1152/ajpcell.00590.2003. [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, Petrovic S, Soleimani M. Expression of the Na+-HCO-3 cotransporter NBC4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol. 2003;284:F41–F50. doi: 10.1152/ajprenal.00055.2002. [DOI] [PubMed] [Google Scholar]
- 4.Groger N, Vitzthum H, Frohlich H, Kruger M, Ehmke H, Braun T, Boettger T. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet. 2012;21:1025–1036. doi: 10.1093/hmg/ddr533. [DOI] [PubMed] [Google Scholar]
- 5.Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO cotransporter (NBCe2) Am J Physiol Cell Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- 6.Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium-bicarbonate cotransporter. Am J Physiol Cell Physiol. 2002;282:C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- 7.Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, Turner ST, Weder AB, Boerwinkle E. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension. 2004;43:477–482. doi: 10.1161/01.HYP.0000111585.76299.f7. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, Hopkins PN. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension. 2006;47:532–536. doi: 10.1161/01.HYP.0000196949.26088.3c. [DOI] [PubMed] [Google Scholar]
- 9.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leviel F, Hubner CA, Houillier P, Morla L, El MS, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, Moore KJ. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 12.Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 13.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci U S A. 2009;106:11800–11805. doi: 10.1073/pnas.0904635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen D, Cornelius RJ, Rivero-Hernandez D, Yuan Y, Li H, Weinstein AM, Sansom SC. Relation between BK-alpha/beta4-mediated potassium secretion and ENaC-mediated sodium reabsorption. Kidney Int. 2014;86:139–145. doi: 10.1038/ki.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-alpha expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol. 2013;305:F463–F476. doi: 10.1152/ajprenal.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Giebisch G. Tubule function in transgenic mice. Exp Nephrol. 1998;6:447–453. doi: 10.1159/000020554. [DOI] [PubMed] [Google Scholar]
- 17.Vallon V. Micropuncturing the nephron. Pflugers Arch. 2009;458:189–201. doi: 10.1007/s00424-008-0581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwark JR, Jansen HW, Lang HJ, Krick W, Burckhardt G, Hropot M. S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflugers Arch. 1998;436:797–800. doi: 10.1007/s004240050704. [DOI] [PubMed] [Google Scholar]
- 19.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 20.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin G, Ren H, Chen X, Zhai XY. Computer-assisted 3D Reconstruction of Mouse Connecting Tubule. Journal of China Medical University. 2010;39:401–403. [Google Scholar]
- 22.Lin SH, Cheema-Dhadli S, Chayaraks S, Chen CB, Gowrishankar M, Halperin ML. Physiological disposal of the potential alkali load in diet of the rat: steps to achieve acid-base balance. Am J Physiol. 1998;274:F1037–F1044. doi: 10.1152/ajprenal.1998.274.6.F1037. [DOI] [PubMed] [Google Scholar]
- 23.Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW, Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013;24:1053–1062. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 25.Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3- transporter NBCn1-mediated HCO3- influx in rat medullary thick ascending limb. J Physiol. 2004;555:205–218. doi: 10.1113/jphysiol.2003.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver RB, Frindt G, Windhager EE, Palmer LG. Feedback regulation of Na channels in rat CCT. I. Effects of inhibition of Na pump. Am J Physiol. 1993;264:F557–F564. doi: 10.1152/ajprenal.1993.264.3.F557. [DOI] [PubMed] [Google Scholar]
- 27.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 28.Rossier BC. Epithelial sodium channel (ENaC) and the control of blood pressure. Curr Opin Pharmacol. 2014;15:33–46. doi: 10.1016/j.coph.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Rossier BC, Schild L. Epithelial sodium channel: mendelian versus essential hypertension. Hypertension. 2008;52:595–600. doi: 10.1161/HYPERTENSIONAHA.107.097147. [DOI] [PubMed] [Google Scholar]
- 30.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol. 2005;16:3154–3159. doi: 10.1681/ASN.2005050460. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Jr, Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov TS, Levchenko V, O'Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW, Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013;24:1053–1062. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hye Khan MA, Pavlov TS, Christain SV, Neckář J, Staruschenko A, Gauthier KM, Capdevila JH, Falck JR, Campbell WB, Imig JD. Epoxyeicosatrienoic acid analogue lowers blood pressure through vasodilation and sodium channel inhibition. Clin Sci (Lond) 2014;127:463–474. doi: 10.1042/CS20130479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mironova E, Boiko N, Bugaj V, Kucher V, Stockand JD. Regulation of Na excretion and arterial blood pressure by purinergic signalling intrinsic to the distal nephron: consequences and mechanisms. Acta Physiol (Oxf) 2015;213:213–221. doi: 10.1111/apha.12372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.