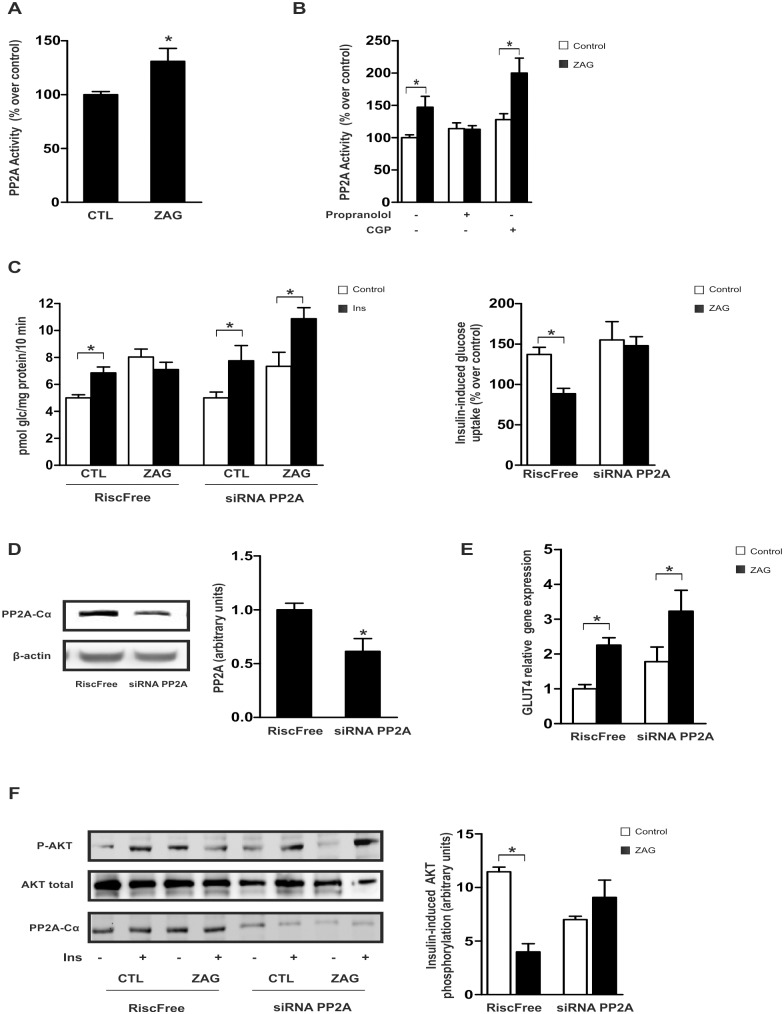
Fig 4. ZAG activation of PP2A phosphatase impairs insulin-stimulated glucose uptake and AKT phosphorylation in SGBS adipocytes.
(A) Differentiated human adipose cells were cultured with 25 μg/ml ZAG for 24 hours and PP2A activity was measured. (B) PP2A activity was measured in mature adipocytes pre-treated with 1 μM propranolol or 300 nM CPG20712A (CPG) prior to culture with ZAG. Data are presented as mean±SEM (n = 3). *, P < 0.01 vs control. (C) Adipose cells were transfected with 100 nM siRNA against the α-catalytic subunit of PP2A (PP2A-Cα) or RISC-free (control cells) and cultured with or without 25 μg/ml ZAG for 24 hours prior to stimulation with 100 nM insulin (Ins) for 30 minutes. Glucose uptake was measured by incorporation of labelled 2-deoxyglucose into the cells during the last 10 minutes of culture. Results from 3 independent experiments performed in triplicate are expressed as pmol glc/mg prot/10 min. Right panels represent percentage of stimulation produced by insulin over control cells (no insulin, without or with ZAG, respectively). *, P < 0.01. (D) PP2A-Cα protein expression was analyzed by western blot in control and transfected cells. A representative experiment is shown together with densitometric analysis (3 independent experiments). *, P < 0.01. (E) GLUT4 mRNA levels were analyzed by qPCR in siRNA control and PP2A-Cα-silenced adipocytes in the absence or presence of ZAG. Data are presented as mean±SEM (n = 3). *, P < 0.01 vs control. (F) Cells transfected as in D were cultured or not with 25 μg/ml ZAG for 24 hours prior to stimulation with 100 nM insulin (Ins) for 15 minutes. Cell lysates were analyzed by western blotting using antibodies against phosphorylated and total Akt (Ser473). A representative experiment is shown together with densitometric analysis of phosphorylated vs total proteins (3 independent experiments). *, P < 0.01.