Figure 1. Calcium-independent dimerization and calcium-dependent self-aggregation of profilaggrin N-terminal S-100 fused-type calcium-binding domain.
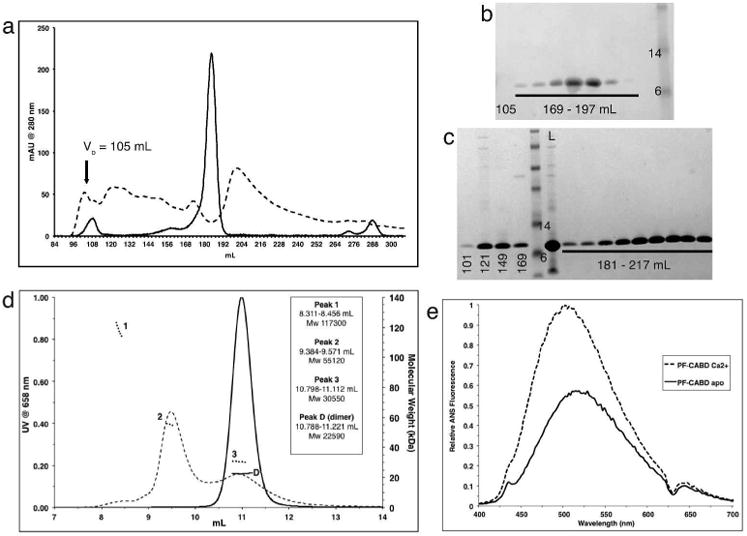
Gel filtration (GF) of PF-CABD with 1mM EDTA (solid line) produced a single peak [180-191mL], but with 5mM CaCl2 (dotted line) produced multiple high MW aggregates [100-186mL]. Void volume (Vo). (b) SDS-PAGE of GF eluted protein (1mM EDTA) within 169-197mL fractions demonstrated PF-CABD protein; none was present in 105mL fraction. (c) SDS-PAGE of GF eluted protein (5mM CaCl2) in 101-169mL fractions demonstrated PF-CABD high MW L = loaded sample. (d) Light scattering of PF-CABD with 1mM EDTA (solid lines) demonstrated a single homogenous peak with MW (22,590 Da) of a PF-CABD dimer (scattering distribution labeled “D”), but with 5mM CaCl2 (dotted lines) demonstrated multiple peaks of higher than expected MW (scattering distributions labeled 1-3). (e) Increased ANS fluorescence emission for PF-CABD (2 μM) in 2mM CaCl2 (dotted line) compared to PF-CABD in 2mM EDTA (solid line) indicated calcium-dependent structural opening of the protein to expose hydrophobic residues.
