Figure 2. Crystal structure of the N-terminal S100 fused-type calcium-binding domain of human profilaggrin.
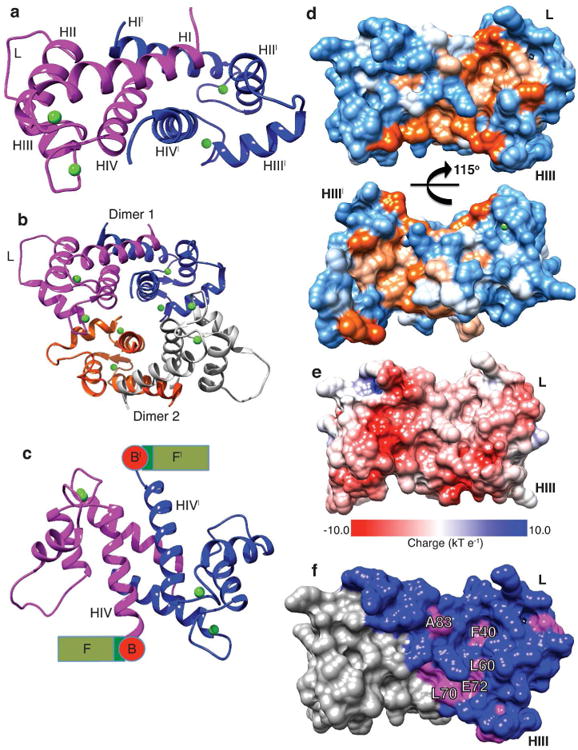
(a) PF-CABD is biologically a dimer (protein 1, magenta; protein 2, blue with prime symbol). PF-CABD monomer forms a four-helix bundle. (b) The crystal AU contained two PF-CABD dimers (dimer 1, magenta/blue; dimer 2, orange/gray). The tetramer core is composed of four helix IV helices. (c) PF-CABD dimer rotated forward 120° about x-axis compared to panel 2a to display the antiparallel helix IV plane connected to a schematic of remaining profilaggrin sequence. B = B domain; F = filaggrin units. (d) Molecular surface of hydrophobic pocket in PF-CABD dimer illustrating two hydrophobic pockets (orange color gradient based on degree of hydrophobicity); polar residues = blue; orientation 40° backward rotation about x-axis compared to Figure 2a. (e) Electrostatic surface potential of PF-CABD dimer, demonstrating acidic calcium-binding loops (red) and uncharged pocket (white). Basic residues = blue. (f) Only 5 residues are conserved in the hydrophobic pocket (labeled, magenta) across S100 fused-type protein family. Non-conserved residues colored blue. Calcium ions = green. L = interhelical linker; H = helix.
