Figure 3. The profilaggrin S100 domain interacts with the N-terminus of annexin II.
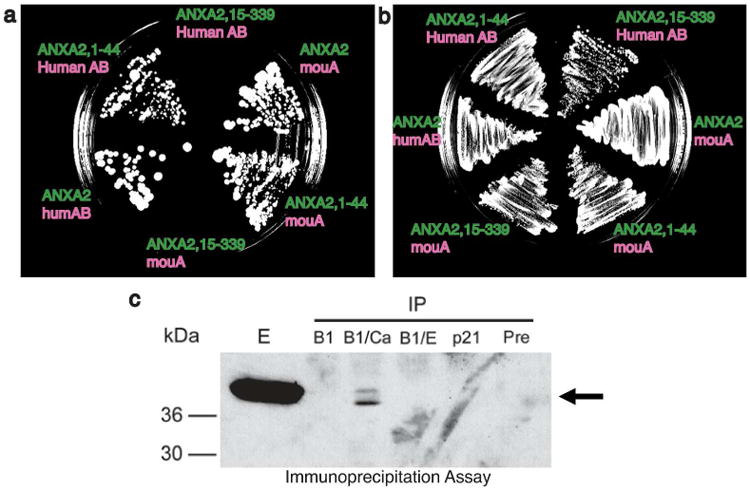
Yeast two-hybrid analysis (panels a and b) demonstrated the A (S100) domain of profilaggrin interacts with the N-terminus of annexin II. (a) Bait (green) and prey (pink) plasmid combinations were plated on HLT-deficient media containing 10 mM 3-amino-1,2,4-triazole. Both full length ANXA2 and a truncated N-terminal protein (ANXA2, 1-44) interacted with human and mouse profilaggrin N-terminus, but an ANXA2 protein lacking the first 14 amino acids (ANXA2, 15-339) showed no Y2H signal. (b) Control (LT-deficient) plate showing confluent growth of bait/prey combinations. (c) Association of profilaggrin N-terminus and annexin II in vitro is calcium-dependent. Epidermal proteins were immunoprecipitated with either profilaggrin B domain (B1) antibody, a p21/WAF1 antibody, or a pre-immune rabbit control. Immunoprecipitated proteins were separated on SDS/polyacrylamide gels and immunblotted with annexin II antibody. The lanes show immunoprecipitation with: B1 antibody, with no additions; B1/Ca, B1 antibody with the addition of 5 mM CaCl2; B1/E, B1 antibody with the addition of 5 mM EDTA; p21/WAF1 antibody or pre-immune serum, with no additions. E represents a control epidermal extract to show annexin II.
