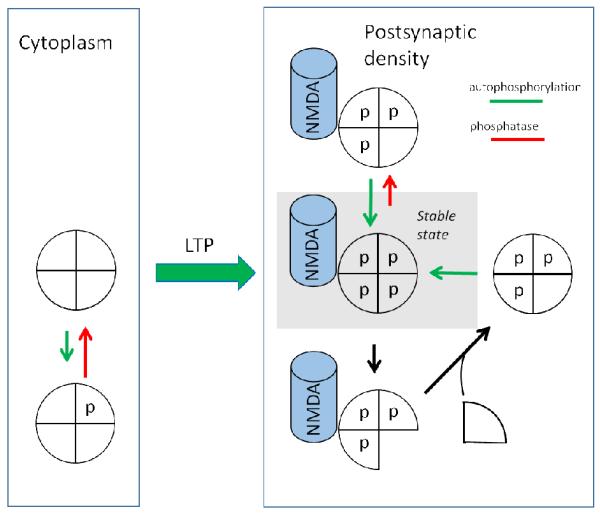
Fig.2. Contribution of autophosphorylation and subunit exchange to maintenance of the on state.
(Left) Simplified model of CaMKII holoenzyme with only four shown subunits, each with a t286 site unphosphorylated. Such holoenzymes are in the cytoplasm, usually bound to actin. (Right) Upon activation during LTP induction, CaMKII translocates to the postsynaptic density, where it binds to the NMDAR. In this on state, each T286 site is phosphorylated. Two types of reactions must be counteracted to make the switch stable. (Right/Upper) Phosphatase may dephosphorylate a subunit; this is counteracted by autophosphorylation of that site by a neighboring active subunit. (Right/Lower) If protein turnover occurs by subunit exchange, a phosphorylated subunit may be replaced by an unphosphorylated subunit. This subunit is then phosphorylated by a neighboring subunit.