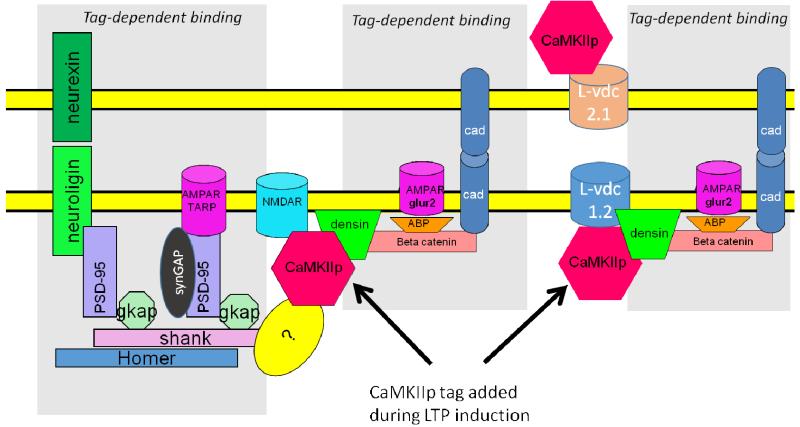
Fig.8. Molecular model of trans-synaptic structural unit.
Activated (phosphorylated) CaMKII binds to the NMDAR, forming the tag. Then other proteins, some newly synthesized, gradually (over the time course of an hour) bind to the synapse in a tag-dependent fashion. Three potential scenarios (not mutually exclusive) are shown in the three grey areas. The addition of adhesion molecules such as N-cadherin may serve to ensure that as the synapse grows, it has the same size presynaptically and postsynaptically. Although beta catenin is shown in the figure, both delta and beta forms bind to densin (Heikkila et al., 2007) and may thus be part of the structural unit. The figure shows a speculative binding interaction (?) that might explain how CaMKII binding to the NMDAR could serve as a seed for the observed addition of Shank and PSD-95.