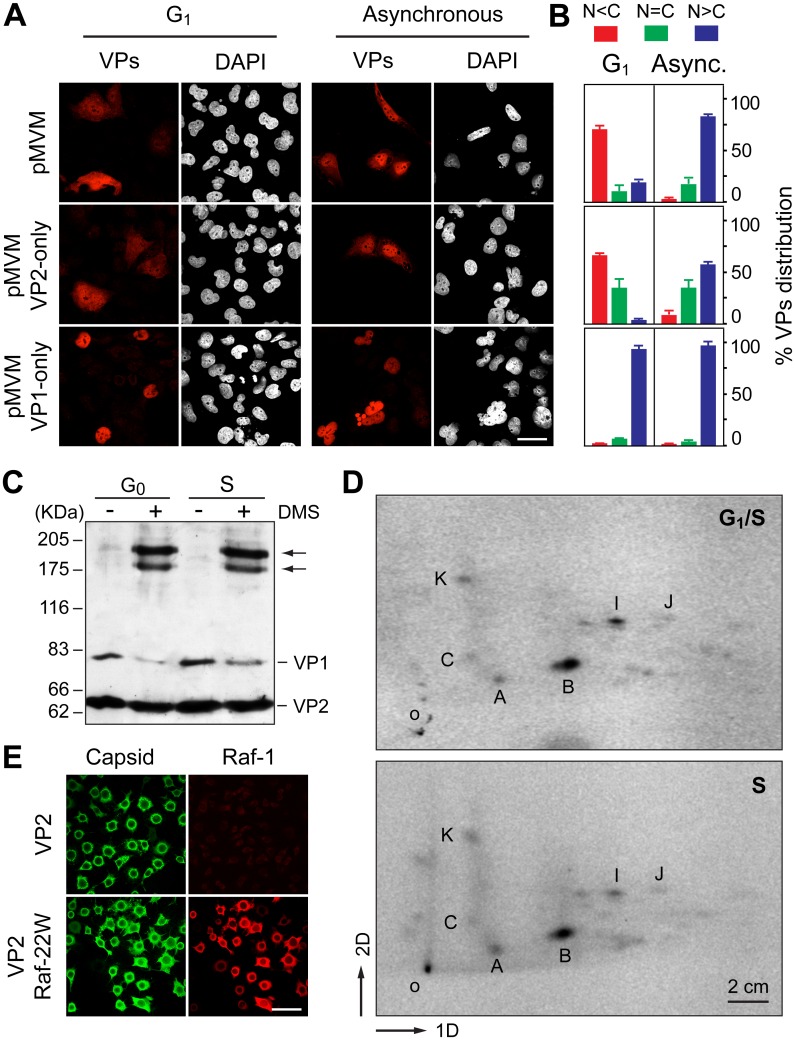
Fig 6. Signals and phosphorylations regulating the nuclear transport of MVM trimeric capsid assembly intermediates.
A. Cell cycle dependence of VP1 and VP2 nuclear accumulation. Confocal images of HFs seeded at high (G1 arrest) or low (asynchronous growth) density, stained with the α-VPs serum upon 48 h postransfection with the indicated genomic MVM plasmids. Scale bar, 25 μm. B. Phenotypes of the VPs subcellular distribution for the indicated plasmids 48 h postransfection in HFs seeded at high (G1 arrest) or low (asynchronous growth) density, stained with the α-VPs antibody based on four independent experiments. C. The VPs assemble into trimers in the cytoplasm of growth arrested mouse cells. Homogenates of MF-VPs made quiescent by serum starvation (G0), or upon 8 h post-stimulation into cycle by serum addition (S), were cross-linked with DMS and analyzed by 5% SDS-PAGE and Western-blot (α-VPs antibody). Arrows indicate the two types of VP trimers [37]. D. Pattern of VP2 phosphorylation in the G1 to S transition. 2D-tryptic phosphopetides analysis of the VP2 protein purified from HF-VPs, 32P-labeled either during isoleucine/aphidicolin arrest (G1/S), or 10 h upon arrest release (S). Phosphopeptides were monitored by phosphoimaging and are named as previously reported [43]. 1D, 2D: first and second dimensions; o, origin. E. Raf-1 phosphorylated VP2 fails to translocate into the nucleus of insect cells. H5 monolayers infected by the Bac-VP2wt alone (upper panels), or co-infecting with the Bac-Raf1-22W (lower panels) baculovirus, stained at 24 hpi with the α-capsid and α-Raf1 antibodies. Scale bar 50 μm.