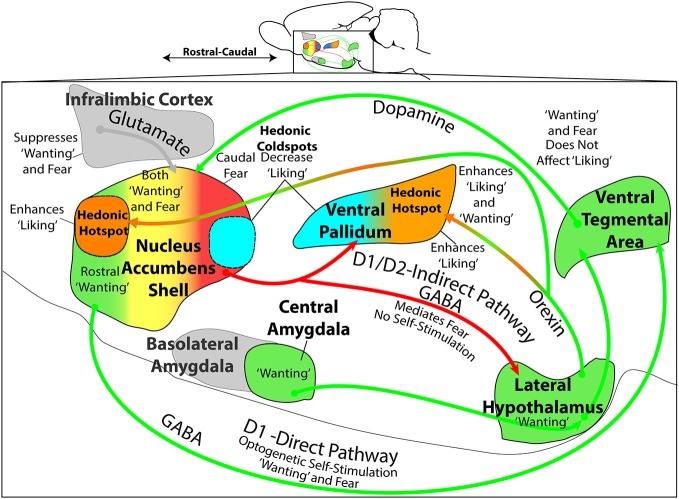
Figure 3.
Mesocorticolimbic-hypothalamic circuitry and functions. Sagittal view depicts structures and circuitry underlying “liking”, “wanting”, and “fear” functions discussed in text. The NAc medial shell contains a hedonic hotspot in the rostral half, where opioid and related stimulation increases “liking” reactions to sucrose taste. Conversely, NAc shell contains a caudal hedonic coldspot, where opioid stimulation suppresses “liking” reactions to sweetness. These functional sites overlap with the NAc shell motivational keyboard for GABAergic/glutamatergic microinjections, in which rostral sites produce desire (e.g., eating, place preference, etc.), caudal sites produce fear (e.g., antipredator reactions, distress calls and bites, and place avoidance). Furthermore, NAc glutamatergic generation of appetitive behavior by DNQX microinjection requires endogenous dopamine stimulation of D1 receptors on direct path neurons that project directly to ventral tegmental area (VTA). Optogenetic stimulation of NAc D1-expressing neurons also supports appetitive self-stimulation behavior throughout the entire medial shell. By contrast, NAc glutamatergic generation of fearful behaviors requires additional dopamine stimulation of D2-receptors, implicating indirect path neurons that project to the VP and lateral hypothalamus (LH). The posterior half of VP contains an opioid hedonic hotspot, whereas the rostral half of VP contains a coldspot where mu opioid stimulation suppresses “liking” reactions to sucrose taste. Optogenetic stimulation of the VP hotspot, or of its lateral hypothalamic inputs, may produce enhanced “liking” and “wanting”. Colors denote implication in “wanting” (green), fear (red), mixed “wanting” and fear (yellow), suppression of “wanting” or “fear” (gray), “liking” (orange), or suppression of “liking” (blue). All data from sources described in text.