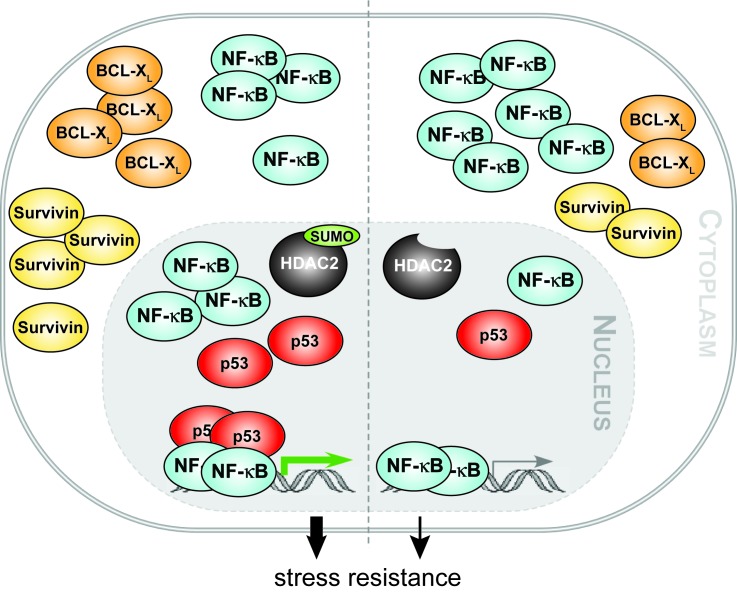
Figure 5. HDAC2 sumoylation integrates NF-κB signaling.
Our work reveals that the epigenetic regulator histone deacetylase 2 (HDAC2) can activate NF-κB-dependent gene expression in human cancer cells and in murine embryonic fibroblasts. We show that the posttranslational modification of HDAC2 with the small ubiquitin-related modifier (SUMO) integrates NF-κB p65 and the tumor suppressor p53. In the absence of HDAC2 sumoylation the activation of NF-κB by HDAC2 is strongly diminished. Our data further suggest that enhanced recruitment of p53 to NF-κB p65 bound to DNA and an increased nuclear accumulation of p65 are causal for an augmented NF-κB-dependent anti-apoptotic gene expression in cells expressing wild-type HDAC2. Additionally, we demonstrate that HDAC2, but not its sumoylation-deficient variant, increases the resistance of colon cancer cells toward genotoxic stress. These findings suggest that the HDAC2-NF-κB signaling node we report in our manuscript contributes to tumorigenesis and chemoresistance.