Figure 7. Pharmacological inhibition of Akt or ectopic expression of constitutively active or dominant negative Akt does not affect cell adhesion.
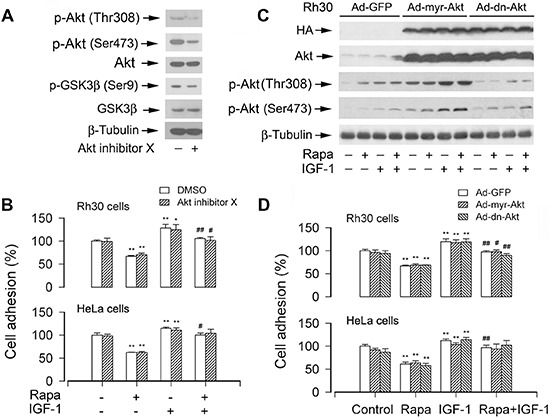
(A) Serum-starved Rh30 were treated with or without Akt inhibitor X (10 μM) for 2 h, followed by Western blot analysis using indicated antibodies, showing that Akt inhibitor X inhibited the phosphorylation of Akt and its substrate GSK3β in the cells. (B) The adhesion of Rh30 and HeLa cells, treated with or without rapamycin and/or IGF-1 following pre-incubation with or without Akt inhibitor X for 2 h, was not significantly affected. (C and D) Serum starved Rh30 and/or HeLa cells, infected with Ad-myr-Akt, Ad-dn-Akt, or Ad-GFP (for control), were treated with or without rapamycin (Rapa, 100 ng/ml) for 2 h, followed by stimulation with or without IGF-1 (10 ng/ml) for 1 h. (C) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (D) Adherent cells were determined using CN IV-coated cell adhesion assay, showing that ectopic expression of myr-Akt or dn-Akt did not exhibit an obvious stimulatory or inhibitory effect on cell adhesion in Rh30 and HeLa cells. Results are means ± SE (n = 12). *P < 0.05, **P < 0.01, difference versus control group; #P < 0.01, ##P < 0.01, difference versus IGF-1 group.
