Fig. 5.
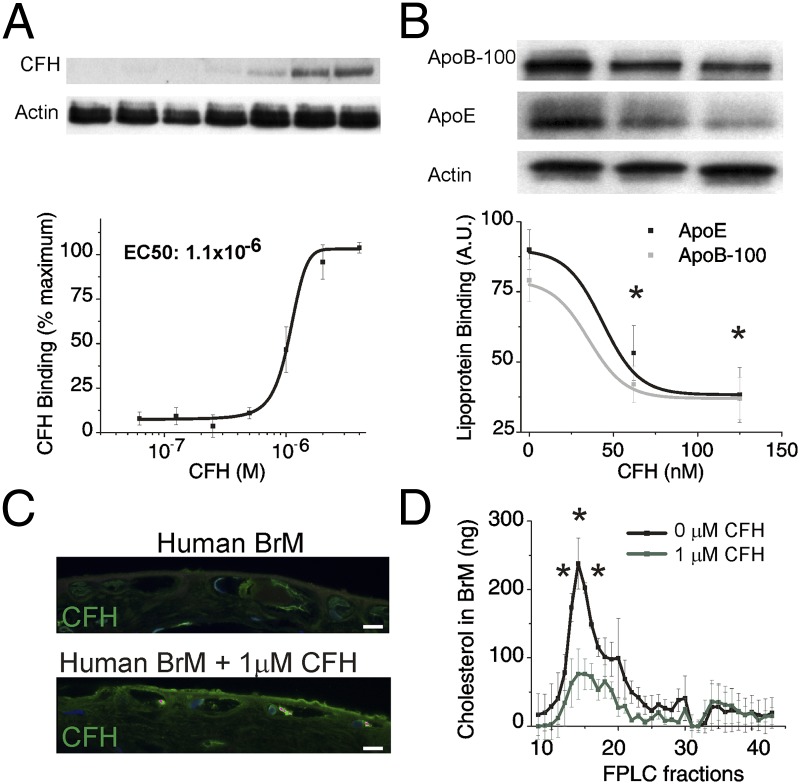
CFH levels regulate lipoprotein binding and remove endogenous human lipoproteins in BrM. (A and B) Six-millimeter porcine RPE/BrM tissue explants were incubated with doubling concentrations of CFH (62 nM to 4 μM) in the presence of a 200 μM excess of albumin. (A) The amount of CFH bound to the tissue explant was determined by Western blot of the tissue lysate to determine EC50 binding. (B) Similar porcine tissue explants were incubated 2 μL of lipoprotein and increasing concentrations of CFH (0, 60, 120 nM), and lipoprotein binding to the explants was assessed by Western blot analysis for ApoB-100 and ApoE. Data are expressed as mean ± SE. The asterisk (*) indicates P < 0.05 for ApoE and ApoB-100 by ANOVA compared with 0 nM CFH. Data presented are a representative experiment from three independent experiments (n = 12–15). (C) Because aged human BrM contains endogenous sub-RPE lipoproteins, we tested the ability of CFH to remove these accumulated lipoproteins ex vivo. Anti-CFH (green) immunohistochemistry of aged human BrM donor tissue, before (Top) and after (Bottom) overnight incubation with 1 μM exogenous CFH, shows accumulation of CFH in BrM. (Scale bar: 5 μm.) (D) FPLC fractionation of human BrM lysates shows endogenous lipoproteins present in aged BrM tissue are removed with the addition of CFH (1 μM CFH, green trace). The asterisk (*) indicates P < 0.05 for total cholesterol in each fraction comparing 0 μM CFH to 1 μM CFH. Three independent experiments each with an n = 3 were performed to confirm these results of human BrM FPLC experiments. Actin served as a loading control in A and B.