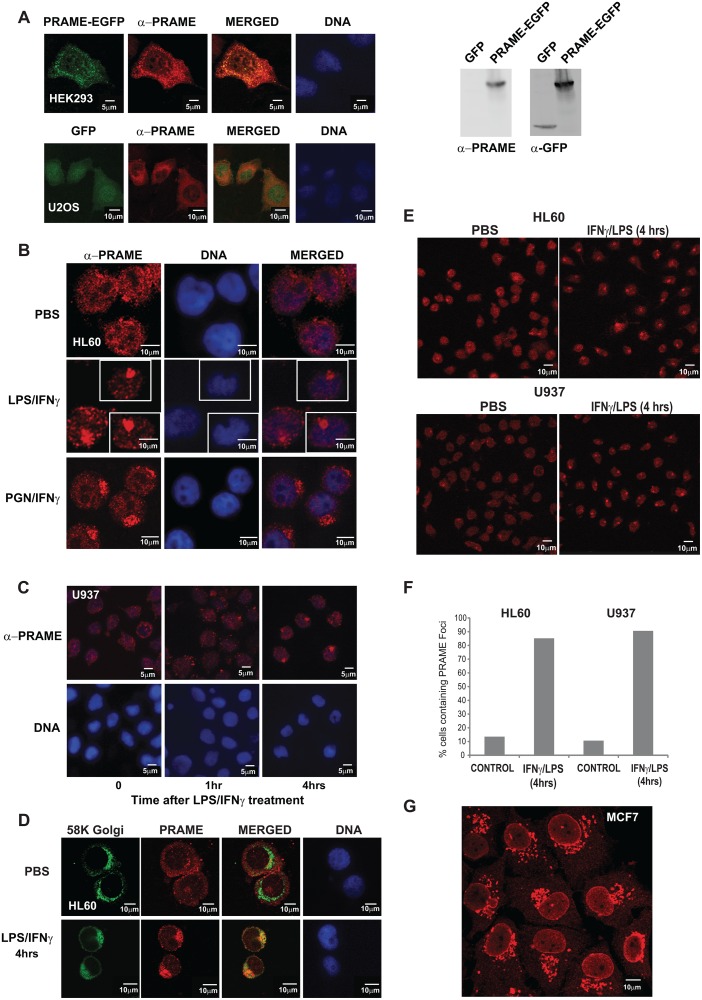
Fig 2. PRAME localises to the Golgi network following LPS/IFNγ treatment.
(A) HEK293 cells (upper panels) were transiently transfected with PRAME-EGFP (green) and stained with α-PRAME antibody (red) to confirm the identity of the overexpressed EGFP fusion protein. U2OS cells (lower panels) were cotranfected with GFP (green) and PRAME-FLAG (red). Merged images indicate the extent of coincidence of the EGFP and α-PRAME signals, and nuclear DNA is indicated (blue). The right hand panels are western blots showing detection of GFP or PRAME-EGFP proteins in whole cell extracts of transfected U2OS cells. (B) Immunostaining of endogenous PRAME in HL60 cells using α-PRAME antibody following treatment with PBS, LPS/IFNγ or PGN/IFNγ for 4 hrs. (C) Immunostaining of endogenous PRAME in U937 cells with α-PRAME following treatment with LPS/IFNγ for 0, 1 and 4 hrs. (D) HL60 cells treated with LPS/IFNγ for 4 hrs and immunostained with α-Golgi 58K (green) and α-PRAME (red). Merged images show the extent of colocalisation of both proteins. For immunofluorescence (A–D), nuclear DNA was stained using Hoechst 33258 and images were captured using a LSM510 confocal laser scanning microscope. (E) Immunostaining of endogenous PRAME in HL60 cells using α-PRAME antibody following treatment with PBS or LPS/IFNγ for 4 hrs. (F) Quantification (n = 60) of the percentage of cells in (E) containing PRAME cytoplasmic foci in treated cells or controls. (G) Immunostaining of endogenous PRAME in MCF-7 cells using α-PRAME antibody.