Figure 2. Abrogation of EGFR-STAT1 signaling can contribute to the mechanism of MUC4 regulation.
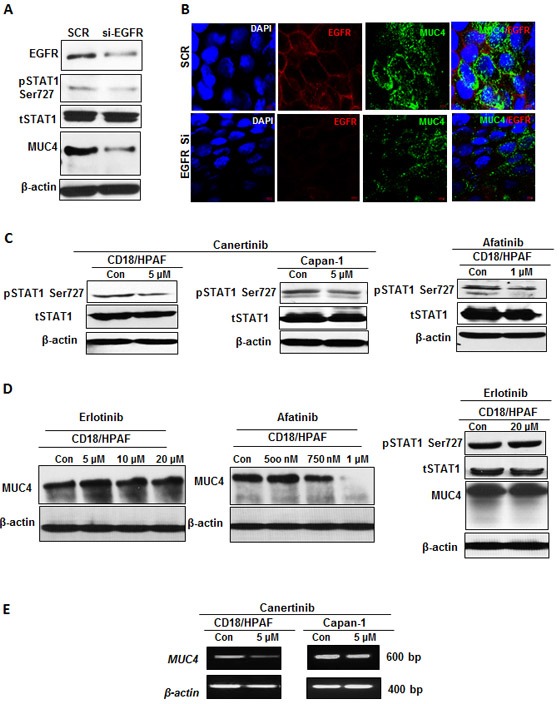
(A). Total cellular lysates harvested form CD18/HPAF cells transiently transfected with EGFR specific siRNA or non-targeted (scramble) control was subjected to western blot analysis, with anti-EGFR, anti phospho-STAT1, anti-STAT1, anti-MUC4 and anti-β-actin antibodies. Total EGFR antibody confirms EGFR silencing in the total protein extracts harvested from CD18/HPAF cells, 96 hour following transfection. (B). Confocal analysis of MUC4 and EGFR co-occurrence in EGFR transiently knockdown CD18/HPAF cells. After performing EGFR specific transient knockdown in CD18/HPAF cells for 96h, knockdown cells were further analyzed with co-localization studies for direct MUC4 inhibition through EGFR-STAT pathway. (C). Western blot analysis of total protein lysates harvested from CD18/HPAF and Capan-1 pancreatic cells, untreated or treated with 5 μM concentration of canertinib and 1 μM concentration of afatinib, alone for 24 hour, using phospho-STAT1, total STAT1 and anti-β-actin antibodies. The mechanism by which phospho-STAT1 is inhibited under pan-EGFR inhibitors treatment is directly correlated with MUC4 regulation. (D). Immunoblotting analysis of MUC4 protein expression upon increasing concentrations of erlotinib and afatinib in CD18/HPAF cells. MUC4 protein and its regulatory transcription factor phospho STAT1 is not inhibited even at higher concentration of erlotinib. Whereas, the level of MUC4 protein expression was decreased in a dose dependent manner, with significant inhibition starting at 750 nM and 1 μM concentration of afatinib compared to vehicle treated controls. (E). RT-PCR was performed on pancreatic cancer cells as described in materials and methods. The representative gel images depict decreased MUC4 transcripts in response to canertinib treatment as compared to vehicle control.
