Abstract
The purpose of the present study was to investigate the expression of TAp73 and ΔNp73 in cervical squamous cancer cells, and to evaluate the prognostic significance of TAp73 and ΔNp73 expression in patients with International Federation of Gynecology and Obstetrics (FIGO) stage I-II cervical squamous cell carcinoma (SCC). The immunohistochemical expression of TAp73 and ΔNp73 was evaluated in 59 FIGO stage I-II cervical SCC tumor samples. Correlations with clinicopathological characteristics were determined by χ2 test. The prognostic impact of TAp73 and ΔNp73 expression with regard to overall survival (OS) was determined by the Kaplan-Meier method. High TAp73 and ΔNp73 expression was detected in 79.7% (47/59) and 76.3% (45/59) of patients, respectively. The expression of TAp73 and ΔNp73 was not associated with age, FIGO stage, pathological differentiation or lymph node metastasis. The 3-year OS rates associated with low and high TAp73 expression were 75.0 and 83.0%, respectively (χ2=0.33; P=0.568), whereas those associated with low and high ΔNp73 expression were 100.0 and 75.6%, respectively (χ2=3.90; P=0.048). High expression levels of TAp73 and ΔNp73 were frequently observed in the cervical squamous cancer cells. Overall, high expression levels of ΔNp73 may indicate an unfavorable prognosis in patients with early-stage cervical SCC.
Keywords: cervical cancer, p73, prognosis
Introduction
Cervical cancer is the second most prevalent female cancer worldwide, and 80–90% of cervical cancers are classified as squamous cell carcinoma (SCC) based on pathological findings (1,2). The majority of patients with early-stage cervical cancer can be treated with radical surgery or radiotherapy (3). Komaki et al (4) reported 5-year overall survival (OS) rates of 89.4 and 79.3% in patients with International Federation of Gynecology and Obstetrics (FIGO) stage I and II cervical cancer, respectively. Pathological factors, including histological type, tumor diameter, lymph node metastasis, lymph vascular space invasion, depth of stromal invasion and parametrial extension, are currently considered independent prognostic factors for survival (5). However, there is no consensus regarding the role of these risk factors for the individual patient (6). The identification of novel markers to accurately predict the prognosis of patients with cervical cancer is therefore necessary.
The transcription factor p53 and its two homologs, p73 and p63, form the p53 gene family. These three genes encode proteins with high similarity at the structural and functional level (7,8). The p53 gene is the prototype tumor suppressor in human cancers due to its proapoptotic and antiproliferative functions in response to oncogenic stress. The p53 gene is mutated and its function is lost in ∼50% of human cancers (9,10). Despite its significant homology to p53, p73 is not a classic Knudson-type tumor suppressor gene, and it has several complex isoforms with opposing functions, including transactivation domain p73 (TAp73) and p73 with inhibitory proteins lacking TA (ΔTAp73). ΔTAp73 is expressed in four different forms as follows: ΔNp73, ΔN'p73, ΔEx2p73 and ΔEx2/3p73, of which ΔNp73 is the predominant form. ΔNp73 is a potent transdominant inhibitor of TAp73 and wild-type p53. The p73 locus encodes a tumor suppressor (TAp73) and a putative oncogene (ΔNp73) (11,12).
Recent studies have demonstrated that TAp73 and ΔNp73 are overexpressed in a number of solid tumors, including lung, ovarian, hepatocellular, breast and colon cancers, and their expression levels are associated with prognosis in patients with these cancers (10–14). However, few studies have investigated the expression levels of TAp73 and ΔNp73 and their prognostic significance in cervical cancer, particularly in early-stage cervical SCC (15). In the current study, the expression of TAp73 and ΔNp73 was investigated in cervical squamous cancer cells, and their prognostic significance was evaluated in patients with FIGO stage I-II cervical SCC.
Patients and methods
Patients and specimen selection
Paraffin-embedded post-operative tissue samples were obtained from the archives of the Department of Pathology, the Second Affiliated Hospital of Soochow University (Suzhou, Jiangsu, China), between January 2009 and December 2010. A total of 59 tumor samples were retrospectively retrieved from patients with FIGO stage I-II cervical SCC. Approval for the current project was obtained from the Ethics Committee of the Second Affiliated Hospital of Soochow University.
The main characteristics of the 59 patients were summarized in Table I. The median age of the patients was 42 years (range, 22–68 years). According to the FIGO stage, the cohort consisted of 44 patients with stage I and 15 patients with stage II SCC. All patients underwent radical surgery. A total of 7 patients with a post-operative pathological diagnosis of pelvic lymph node metastasis received radiotherapy. The target volume included the whole pelvis, and radiation was delivered in four or two fields (anterioposterior and posteroanterior beams). A total dose of 5,000 cGy was delivered in fractions of 200 cGy/day for 5 days per week. Systemic adjuvant treatment was administered to 8 patients; these patients received 80 mg/m2 cisplatin and 135 mg/m2 Taxol every 3 weeks for 2–4 cycles.
Table I.
Patient characteristics.
| Characteristic | Value |
|---|---|
| Patients, n (%) | 59 (100.0) |
| Median age (range), years | 42 (22–68) |
| Pathological diagnosis, n (%) | |
| Cervical squamous cell carcinoma | 59 (100.0) |
| FIGO stage, n (%) | |
| I | 44 (74.6) |
| II | 15 (25.4) |
| Radical surgery, n (%) | 59 (100.0) |
| Post-operative radiotherapy, n (%) | 7 (11.9) |
| Post-operative chemotherapy, n (%) | 8 (13.6) |
FIGO, International Federation of Gynecology and Obstetrics.
Immunohistochemistry
Three serial slides, each 3-µm thick, were cut from paraffin-embedded tissues. One slide was used for hematoxylin/eosin staining and the other two were subjected to immunohistochemical staining using the two-step procedure. Mouse monoclonal anti-human TAp73 antibody (IMG-246; dilution, 1:100; Imgenex, Novus Biologicals, Littleton, CO, USA) and mouse monoclonal anti-human ΔNp73 antibody (ab13649; dilution, 1:100; Abcam, Cambridge, MA, USA) were used. Following deparaffinization at 65°C and hydration, the slides were subjected to antigen retrieval by pressure-cooking for 5 min at 120°C. Endogenous peroxidase activity was neutralized using peroxide block placement on the slides for 15 min at room temperature. The slides were then incubated with anti-TAp73 and anti-ΔNp73 monoclonal antibodies for 30 min at 4°C, followed by incubation with peroxidase-conjugated polymers (ChemMate EnVision/HRP; Gene Tech, Shanghai, China) for 30 min at room temperature. The chromogen reaction was developed in 3,3′-diaminobenzidine tetrahydrochloride (Gene Tech) for 10 min. Finally, hematoxylin was used as a light nuclear counterstain. The negative control used was an immunoglobulin G2b isotype antibody (Dako, Shanghai, China), ensuring the same concentration of immunoglobulins used in the anti-TAp73 and anti-ΔNp73 antibodies.
Assessment of TAp73 and ΔNp73 expression
All slides were evaluated independently by two experienced pathologists. The intensity of staining was recorded as negative, weak or strong. In accordance with the description in the study by Giatromanolaki et al, only strongly-stained cells were considered positive cells (16). The extent of staining was expressed as a percentage of positive cells to total cells and was recorded after examining all optical fields at ×200 magnification. The mean value was used to score all samples. Cells were classified into four categories according to the percentage of positive-staining cells as follows: 1, 0–24%; 2, 25–49%; 3, 50–74%; and 4, 75–100%. The scoring pattern was defined as follows: 1–2, low expression (<50% positive cells); and 3–4, high expression (≥50% positive cells).
Statistical analysis
Statistical analysis was performed using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA)The correlation between the expression of TAp73 and ΔNp73, and the clincopathological characteristics was examined using the χ2 test. OS rates were determined using the Kaplan-Meier method and log-rank test. OS time was defined from the day of surgery to the day of mortality or last follow-up. For all tests, two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of TAp73 and ΔNp73 in cervical SCC cells
The expression of TAp73 and ΔNp73 was detected in the nucleus and cytoplasm of the cervical SCC cells. High TAp73 and ΔNp73 expression was detected in 79.7% (47/59) and 76.3% (45/59) of patients, respectively (Fig. 1A and B). No significant correlation between TAp73 and ΔNp73 expression was observed (χ2=0.415; P=0.519).
Figure 1.
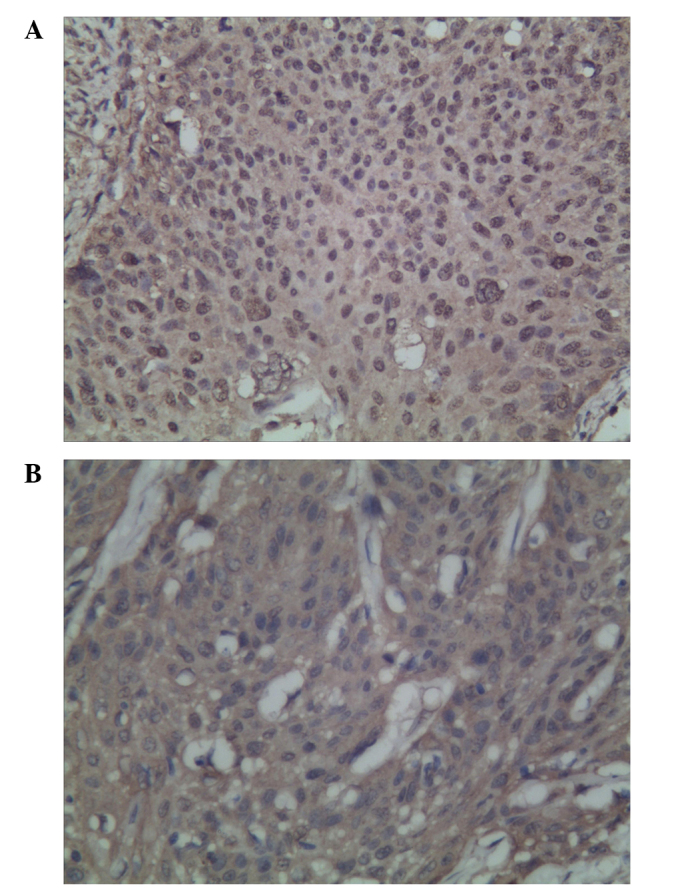
Expression of TAp73 and ΔNp73, as assessed by immunohistochemistry in cervical squamous cell carcinoma cells: (A) High expression of TAp73; and (B) high expression of ΔNp73.
Association of TAp73 and ΔNp73 expression with clincopathological characteristics
Table II shows the associations between the expression levels of TAp73 and ΔNp73 in the 59 patients with FIGO stage I-II cervical SCC and several clinicopathological characteristics. No significant association was observed between TAp73 and ΔNp73 expression and age, FIGO stage, pathological differentiation and lymph node metastasis (Table II).
Table II.
Association of TAp73 and ΔNp73 expression with clincopathological characteristics.
| TAp73 expression, % | ΔNp73 expression, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | No. of patients | Low | High | χ2 | P-value | Low | High | χ2 | P-value |
| Age, years | |||||||||
| ≤42 | 33 | 24.2 | 75.8 | 0.704 | 0.401 | 30.3 | 69.7 | 1.788 | 0.181 |
| >42 | 26 | 15.4 | 84.6 | 15.4 | 84.6 | ||||
| FIGO stage | |||||||||
| I | 44 | 22.7 | 77.3 | 0.609 | 0.435 | 25.0 | 75.0 | 0.155 | 0.694 |
| II | 15 | 13.3 | 86.7 | 20.0 | 80.0 | ||||
| Pathological differentiation | |||||||||
| Low | 12 | 33.3 | 66.7 | 1.600 | 0.449 | 33.3 | 66.7 | 1.215 | 0.545 |
| Medium | 28 | 17.9 | 82.1 | 17.9 | 82.1 | ||||
| High | 19 | 15.8 | 84.2 | 26.3 | 73.7 | ||||
| Lymph node metastasis | |||||||||
| No | 52 | 21.2 | 78.8 | 0.180 | 0.672 | 26.9 | 73.1 | 2.471 | 0.116 |
| Yes | 7 | 14.3 | 85.7 | 0.0 | 100.0 | ||||
FIGO, International Federation of Gynecology and Obstetrics.
Correlation between TAp73 and ΔNp73 expression and overall survival
The average duration of follow-up was 41 months (range, 2–61 months). Analysis of the Kaplan-Meier plots showed that the 3-year OS rate of all patients was 81.4%. The 3-year OS rates in patients with low and high expression levels of TAp73 were 75.0 and 83.0%, respectively (χ2=0.33; P=0.568; Fig. 2A), whereas those in patients with low and high expression levels of ΔNp73 were 100.0 and 75.6%, respectively (χ2=3.90; P=0.048; Fig. 2B).
Figure 2.
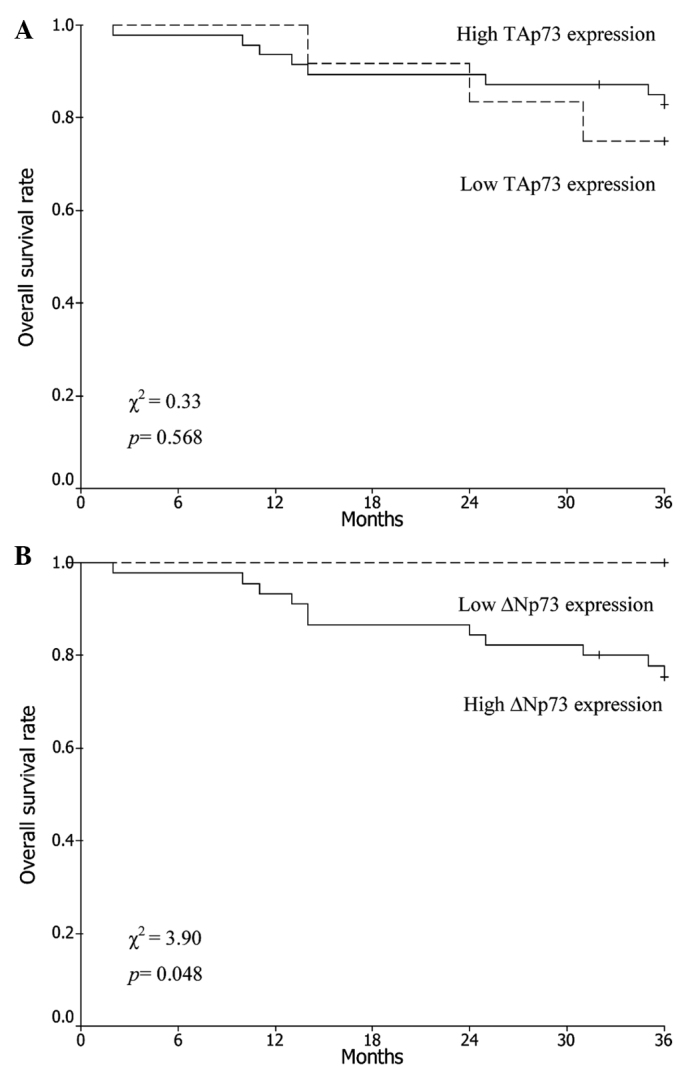
Kaplan-Meier curves for TAp73 and ΔNp73 expression in patients with International Federation of Gynecology and Obstetrics stage I-II cervical squamous cell carcinoma: (A) TAp73 and (B) ΔNp73 expression groups.
Discussion
Previous studies reported that the TAp73 gene mimics p53 suppressor activities, showing proapoptotic effects. However, the ΔNp73 gene was shown to have an antiapoptotic function, in which cooperation with oncogenic RAS induces cell transformation, confers drug resistance and induces the phosphorylation of retinoblastoma protein (11,12,17). The different expression levels of the TAp73 and ΔNp73 isoforms may determine tumorigenesis and resistance to chemo(radio) therapy, as the predominance of ΔNp73 may confer pro-tumorigenic properties (18). A previous study showed that the TAp73 and ΔNp73 isoforms were significantly overexpressed in a number of solid tumors compared with the corresponding normal tissues, suggesting that the balance between these two isoforms may play a role in the regulation of cell proliferation and cell death (19). Hofstetter et al (20) showed that TAp73 and ΔNp73 were expressed in 88.0% (73/83) and 57.8% (48/83) of ovarian cancer samples, respectively. Liu et al (15) reported the positive expression of TAp73 and ΔNp73 in 41.0 and 30.8% of patients with cervical cancer, respectively. Moreover, cancers that expressed a higher level of ΔNp73 tended to express a lower level of TAp73. Müller et al (11) also reported that TAp73 and ΔNp73 are inversely regulated and showed that the high expression of TAp73 is correlated with the low expression of ΔNp73. These results suggest that the expression of the two isoforms is upregulated via different mechanisms in different cancers. In the current study, it was found that 79.7% (47/59) and 76.3% (45/59) of patients with cervical SCC exhibited high expression levels of TAp73 and ΔNp73, respectively. However, no significant correlation was observed between TAp73 and ΔNp73 expression (χ2=0.415; P=0.519).
With respect to the clinical significance of TAp73 expression in cancers, Castellino et al (21) reported that medulloblastoma patients with high expression of TAp73 showed favorable disease-free survival and overall survival times. Similarly, Liu et al (15) showed that TAp73 overexpression predicted a better survival in patients with cervical SCC receiving radiotherapy. However, Hofstetter et al (20) reported that TAp73 expression had no prognostic significance in ovarian cancer. In the present study, the 3-year OS rates in patients with low and high expression levels of TAp73 were 75.0 and 83.0%, respectively, in patients with FIGO stage I-II cervical SCC after radical surgery (χ2=0.33; P=0.568). Further study is required to determine the prognostic significance of TAp73 expression.
Emerging evidence suggests that ΔNp73, rather than TAp73, is the main physiologically relevant component of tumor-associated p73 overexpression and that it functionally overrides the frequent concomitant increase in TAp73. Several studies have shown that a high expression level of ΔNp73 is correlated with tumor progression and poor survival rates in a number of cancer types. Uramoto et al (9) showed that ΔNp73 expression in lung cancer is not correlated with clinicopathological factors, including histological type, pathological tumor stage and node stage. However, lung cancer patients with high ΔNp73 expression exhibited a lower 5-year survival rate than those with low ΔNp73 expression. Similarly, Müller et al (11) reported that a high expression level of ΔNp73 is correlated with reduced survival in hepatocellular carcinoma patients. Liu et al (15) found that ΔNp73 overexpression is significantly associated with resistance to radiotherapy, disease recurrence and poor survival in patients with cervical SCC. These findings indicate that ΔNp73 may be a potential marker for predicting the prognosis and sensitivity to radiotherapy in patients with cervical SCC. In the current study, it was shown that the 3-year OS rates of FIGO stage I-II cervical SCC patients with low and high expression levels of ΔNp73 were 100.0 and 75.6%, respectively, following radical surgery (χ2=3.90; P=0.048). Taken together, these findings suggest that ΔNp73 is associated with an unfavorable prognosis due to its role in chemo(radio) therapy resistance and tumor aggressiveness.
In conclusion, TAp73 and ΔNp73 were frequently overexpressed in the cervical SCC cells in the present study. High expression of ΔNp73 may indicate an unfavorable prognosis in early-stage cervical SCC. However, this retrospective study was potentially limited by the relatively small number of patients. Additional larger studies are required to reach a definitive conclusion.
Acknowledgements
This study was supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant no. PAPD201105) and Jiangsu Province's Key Medical Department in 2011 (grant no. RC2011144).
References
- 1.Lee MY, Shen MR. Epithelial-mesenchymal transition in cervical carcinoma. Am J Transl Res. 2012;4:1–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Y, Liu JH, Jin L, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010;294:204–210. doi: 10.1016/j.canlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 4.Komaki R, Brickner TJ, Hanlon AL, Owen JB, Hanks GE. Long-term results of treatment of cervical carcinoma in the United States in 1973, 1978 and 1983: Patterns of Care Study (PCS) Int J Radiat Oncol Biol Phys. 1995;31:973–982. doi: 10.1016/0360-3016(94)00489-7. [DOI] [PubMed] [Google Scholar]
- 5.Biewenga P, van der Velden J, Mol BW, et al. Prognostic model for survival in patients with early stage cervical cancer. Cancer. 2011;117:768–776. doi: 10.1002/cncr.25658. [DOI] [PubMed] [Google Scholar]
- 6.Biewenga P, van der Velden J, Mol BW, et al. Validation of existing prognostic models in patients with early-stage cervical cancer. Gynecol Oncol. 2009;115:277–284. doi: 10.1016/j.ygyno.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Bourdon JC. p53 family isoforms. Curr Pharm Biotechnol. 2007;8:332–336. doi: 10.2174/138920107783018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salomoni P, Dyer MJS. Delta N-p73: the enemy within. Cell Death Differ. 2005;12:1553–1554. doi: 10.1038/sj.cdd.4401802. [DOI] [PubMed] [Google Scholar]
- 9.Uramoto H, Sugio K, Oyama T, et al. Expression of the p53 family in lung cancer. Anticancer Res. 2006;26:1785–1790. [PubMed] [Google Scholar]
- 10.Khoury MP, Bourdon JC. p53 isoforms: an intracellular microprocessor? Genes Cancer. 2011;2:453–465. doi: 10.1177/1947601911408893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller M, Schilling T, Sayan AE, et al. TAp73/Delta Np73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ. 2005;12:1564–1577. doi: 10.1038/sj.cdd.4401774. [DOI] [PubMed] [Google Scholar]
- 12.Domínguez G, García JM, Peña C, et al. Delta TAp73 upregulation correlates with poor prognosis in human tumors: putative in vivo network involving p73 isoforms, p53, and E2F-1. J Clin Oncol. 2006;24:805–815. doi: 10.1200/JCO.2005.02.2350. [DOI] [PubMed] [Google Scholar]
- 13.Moll UM. The role of p63 and p73 in tumor formation and progression: coming of age toward clinical usefulness. Clin Cancer Res. 2003;9:5437–5441. [PubMed] [Google Scholar]
- 14.Becker K, Pancoska P, Concin N, et al. Patterns of p73 N-terminal isoform expression and p53 status have prognostic value in gynecological cancers. Int J Oncol. 2006;29:889–902. [PubMed] [Google Scholar]
- 15.Liu SS, Chan KY, Cheung AN, Liao XY, Leung TW, Ngan HY. Expression of deltaNp73 and TAp73alpha independently associated with radiosensitivities and prognoses in cervical squamous cell carcinoma. Clin Cancer Res. 2006;12:3922–3927. doi: 10.1158/1078-0432.CCR-05-2573. [DOI] [PubMed] [Google Scholar]
- 16.Giatromanolaki A, Koukourakis MI, Koutsopoulos A, Chloropoulou P, Liberis V, Sivridis E. High Beclin 1 expression defines a poor prognosis in endometrial adenocarcinomas. Gynecol Oncol. 2011;123:147–151. doi: 10.1016/j.ygyno.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Grob TJ, Fey MF, Tobler A. The two faces of p73. Cell Death Differ. 2002;9:229–230. doi: 10.1038/sj.cdd.4401018. [DOI] [PubMed] [Google Scholar]
- 18.Soldevilla B, Millàn CS, Bonilla F, Domínguez G. The TP73 complex network: ready for clinical translation in cancer? Genes Chromosomes Cancer. 2013;52:989–1006. doi: 10.1002/gcc.22095. [DOI] [PubMed] [Google Scholar]
- 19.Melino G, Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter G, Berger A, Chamson M, et al. Clinical relevance of TAp73 and ΔNp73 protein expression in ovarian cancer: a series of 83 cases and review of the literature. Int J Gynecol Pathol. 2011;30:527–531. doi: 10.1097/PGP.0b013e31821ac519. [DOI] [PubMed] [Google Scholar]
- 21.Castellino RC, De Bortoli M, Lin LL, et al. Overexpressed TP73 induces apoptosis in medulloblastoma. BMC Cancer. 2007;7:127. doi: 10.1186/1471-2407-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]


