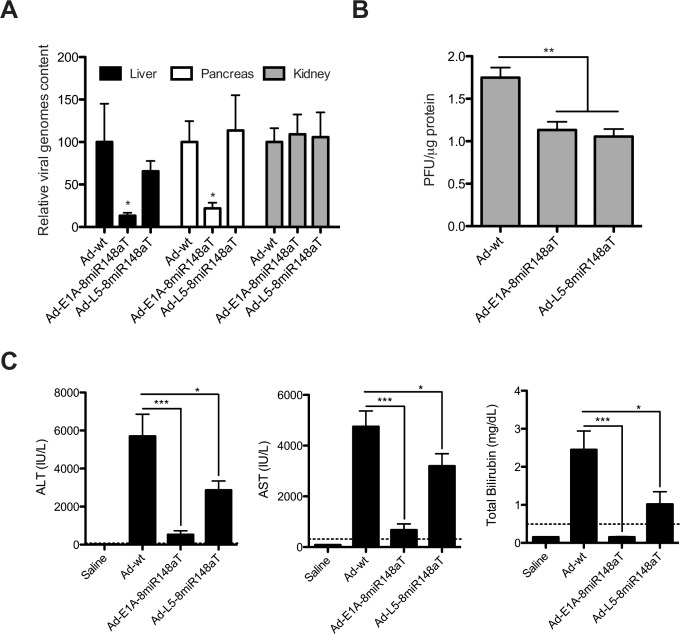
Figure 4. Ad-L5-8miR148aT replication is attenuated in mice liver and displays reduced hepatotoxicity following systemic delivery.
A viral dose of 2×1010vp of Ad-wt, Ad-E1A-8miR148aT or Ad-L5-8miR148aT was intravenously delivered to wild-type mice C57BL/6 mice (n=10). Four days later liver and blood samples were collected. (A). Relative viral replication compared to Ad-wt assessed by genomic qPCR of the L3 gene in liver, pancreas and kidneys (n=10/treatment). * p<0.05. (B). Viral production from livers of mice treated with Ad-wt, Ad-E1A-8miR148aT and Ad-L5-8miR148aT assessed by hexon immunostaining (n=10/treatment). ** p<0.01. (C). Assessment of hepatotoxicity by the determination of AST, ALT and total bilirubin in the serum. Dashed lines correspond to the reference values for C57BL/6 mice. *, ** and *** denote p<0.05, p<0.01 and p<0.001, respectively.