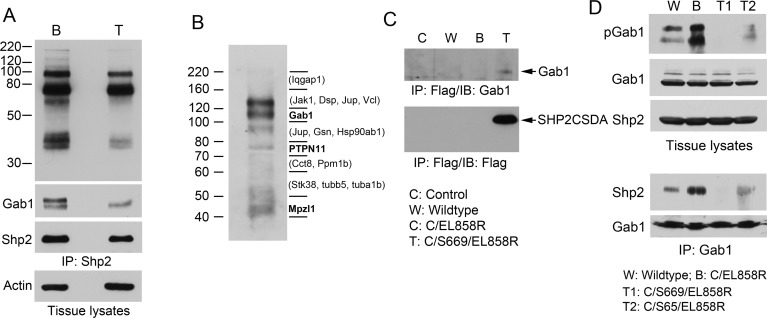
Figure 6. Analysis of Gab1 tyrosine phosphorylation and association with Shp2 in transgenic mouse lung.
A, lung tissue lysates (4 mg/each) of C/EL858R (B) and C/S669/EL858R (T) mice induced with Dox diet for 6 weeks were immunoprecipitated with anti-Shp2. Portions of immunoprecipitates (1/5, 1/5, 1/20) were analyzed by immunoblots with antibodies to phosphotyrosine (PY20), Gab1, and Shp2, respectively. Ten μg of tissue lysates were also analyzed by immunoblot with an antibody to actin. B, Lung tissue lysates from C/S669/EL858R mice induced with Dox diet for 4 weeks were immunoprecipitated with an anti-Flag antibody. One-tenth of immunoprecipitates were analyzed by immunoblotting with an anti-phosphotyrosine antibody (PY20). The rest of immunoprecipitates were separated on a SDS-polyacrylamide gel in the same experiment and gel slices were subjected to trypsin-digestion and protein identification by LC/MS/MS. Bold letters indicate tyrosine phosphorylation residues have been identified on these proteins. Proteins in parentheses are those that known to be tyrosine phosphorylated (based on www.phosphosite.org) that were detected in the indicated gel slices, but their tyrosine-phosphorylated tryptic peptides were not detected in our experiment. C, Mouse lung tissue lysates from indicated genotypes fed with Dox diet for 4 weeks were immunoprecipitated with the anti-Flag antibody and then immunoblotted with indicated antibodies. D, Transgenic mice of indicated genotypes were fed with Dox diet for 4 weeks. Lung tissue lysates were analyzed by immunoblotting with indicated antibodies (top panels). Gab1 was immunoprecipitated from these lung tissues and immunoblotted with antibodies to Shp2 or Gab1 (lower panels).