Figure 2.
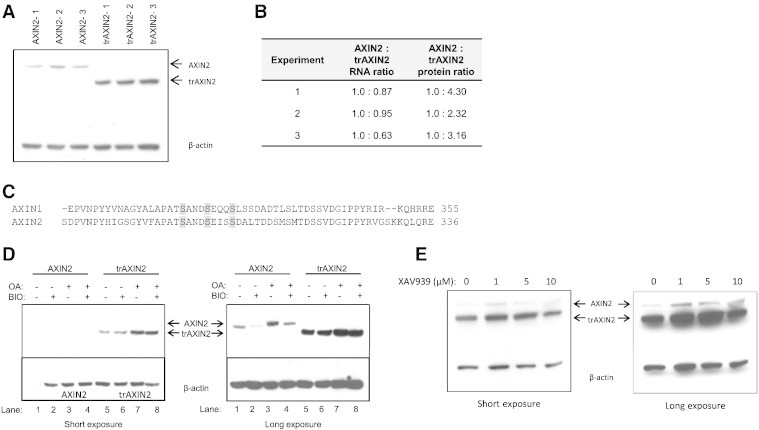
Analysis of AXIN2 and trAXIN2 protein abundance and regulation in transient transfection assays in HEK293T and effects of inhibitors of GSK3β, PP2A, and tankyrase on AXIN2 expression. (A) The trAXIN2 protein is expressed at increased levels in HEK293T cells relative to wild-type AXIN2. HEK293T cells were transfected with equal molar amounts of the AXIN2 or trAXIN2 expression vectors. Protein and RNA were collected for analysis. An IB from protein lysates was prepared from three separate transfection experiments for each of the two constructs, with AXIN2 and trAXIN2 proteins detected by electrochemiluminescence (ECL)-based IBs with an antibody against the FLAG-epitope. (B) Quantification of ectopically expressed AXIN2 transcripts in the transfected HEK293T cells by quantitative RT-PCR using primers in the FLAG sequence and protein levels of AXIN2 protein, based on IB analyses relative to β-actin levels, using ImageJ software. (C) A relevant portion of the AXIN1 and AXIN2 protein sequences are shown, with potential GSK3β phosphorylation sites in the proteins highlighted. (D) IB analysis of AXIN2 and trAXIN2 expression in transiently transfected HEK293T cells ectopically expressing the full-length or trAXIN2 proteins, following a 6-hour BIO and/or OA treatment, with “−” indicating no treatment and “+” indicating treatment. Shorter and longer exposures of the IBs are shown. (E) HEK293T cells were co-transfected with FLAG-tagged AXIN2 and trAXIN2 expression constructs and then treated with increasing doses of XAV939, a tankyrase inhibitor, for 24 hours after transfection, before preparing protein lysates. IB analysis to detect the ectopically expressed AXIN2 and trAXIN2 proteins was carried out with an anti-FLAG antibody.
