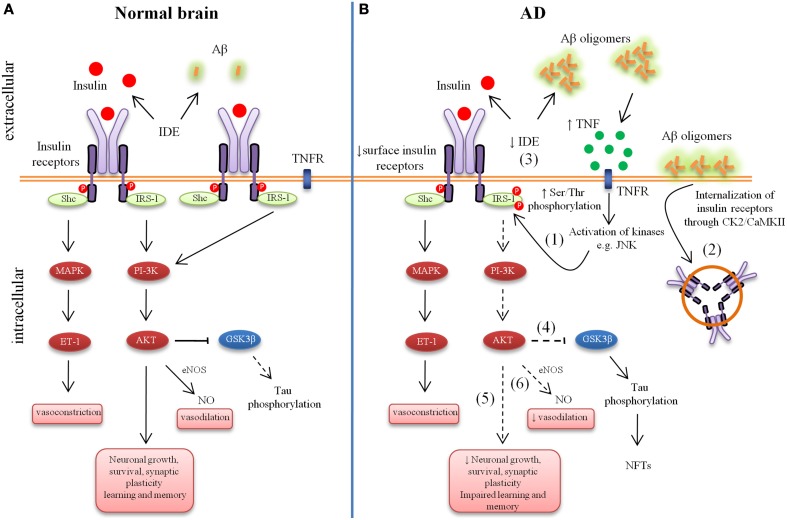
Figure 1.
Aberrant brain insulin signaling in Alzheimer's Disease (AD). Schematic outline of neuronal insulin signaling in the normal brain (A) and AD brain (B). Under physiological conditions, insulin binding to its receptor triggers phosphorylation of insulin receptor substrate-1 (IRS-1). This results in phosphoinositide 3-kinase (PI3K) activation and downstream cellular responses that facilitate neuronal growth, neuronal survival, synaptic plasticity, learning and memory, etc. Activation of the IR can result in both vasodilatation and vasoconstriction and under physiological conditions there is a balance of both processes to regulate the immediate metabolic requirements of various tissues. In AD, accumulation of amyloid-β (Aβ) oligomers leads to increased tumor necrosis factor-alpha (TNF-α) levels and activation of stress kinases such as c-Jun N-terminal kinase (JNK) resulting in inhibitory serine phosphorylation of IRS-1 (1). Aβ oligomers cause removal of IRs from the cell surface mediated by Casein Kinase 2 (CK2) and Ca2+/Calmodulin-Dependent Kinase II (CaMKII) and redistribute them to cell bodies (2). Insulin resistance lowers the expression of Aβ-degrading insulin degrading enzyme (IDE) (3). Lowered IDE expression further decreases the availability of IDE for Aβ degradation. The reduction in brain insulin signaling increases GSK-3β activity (4), which increases abnormal tau phosphorylation. Deficient insulin signaling leads to impairment in nerve growth, synaptic plasticity, learning and memory, etc. (5). Aberrant phosphorylation of IRS causes an imbalance in homeostatic regulation of vascular function (6). This decreased production of NO may results in decreased cerebral blood flow and increased pro-inflammatory cytokines and reactive oxygen species production.