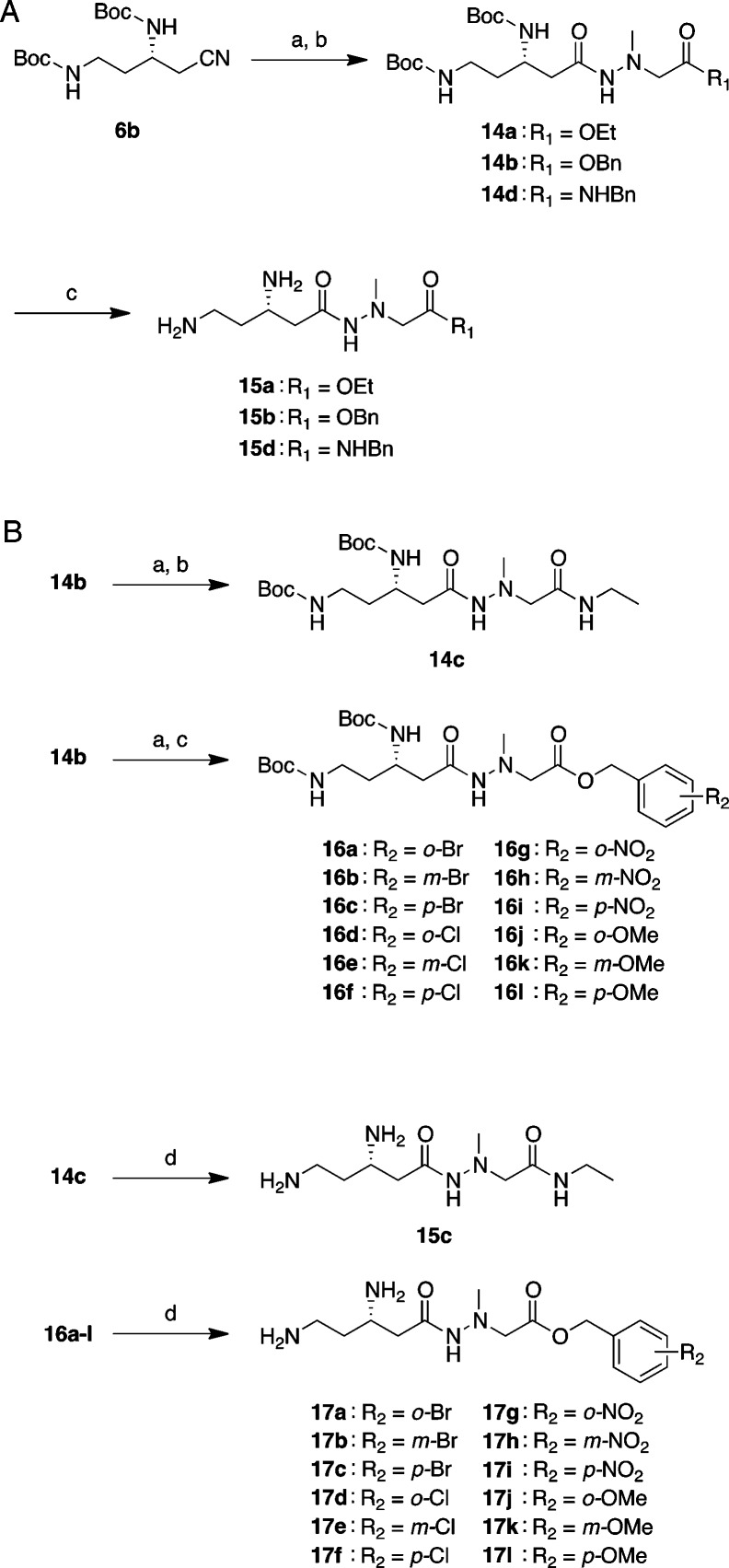
Scheme 2. Synthesis of 15a–d and 17a–l.
Reagents and conditions: (A) Synthesis of 15a–b and 15d: (a) KOH, EtOH/H2O, 80 °C, 45 min–4 h; (b) ethyl or benzyl 2-(1-methylhydrazinyl)acetate 13a,b or N-benzyl-2-(1-methylhydrazinyl)acetamide 13c, HOBt·H2O, Et3N, EDC·HCl, DMF, rt, overnight, 26–57% (2 steps); (c) 4 M HCl/dioxane, rt, 1 h, then, RP-HPLC, 36–50%. (B) Synthesis of 15c and 17a–l: (a) Pd/C, H2, MeOH, rt, 15 min; (b) ethylamine·HCl, HOBt·H2O, Et3N, EDC·HCl, DMF, rt, overnight, 70% (2 steps); (c) substituted benzyl alcohol, DMAP, DCC, rt, overnight, 23–68% (2 steps); (d) 4 M HCl/dioxane, rt, 1 h, then, RP-HPLC, 23–90%.