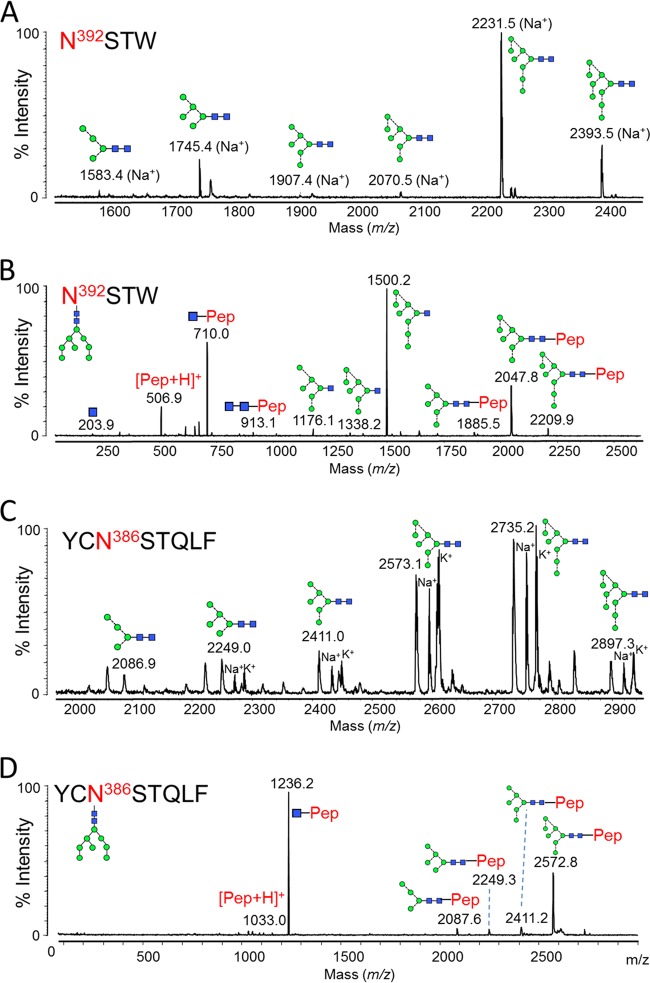
FIG 3.
Glycans present at the Asn392 and Asn386 glycosylation sites. gp120 was digested with chymotrypsin (Promega) before RP-HPLC and MALDI analysis. (A) MALDI MS of Asn392 glycopeptides (NSTW). Sodium, [M + 23]+, and potassium, [M + 39]+, adducts were observed: both peaks were used for measuring abundances (Table 1). (B) MALDI MS/MS fragmentation spectrum of the peak corresponding to the Man8GlcNAc2 glycopeptide. Fragmentation peaks corresponded to the protonated masses. (C) MALDI MS of Asn386 glycopeptides (YCNSTQLF). The cysteine was modified due to treatment with iodoacetamide (carbamidomethyl, +57). Protonated glycopeptides, as well as sodium and potassium adducts, were detected: all were used for calculation of abundances (Table 1). (D) MALDI MS/MS fragmentation spectrum of the peak corresponding to the Man8GlcNAc2 glycopeptide. Fragmentation peaks corresponded to the protonated masses.