FIG 3.
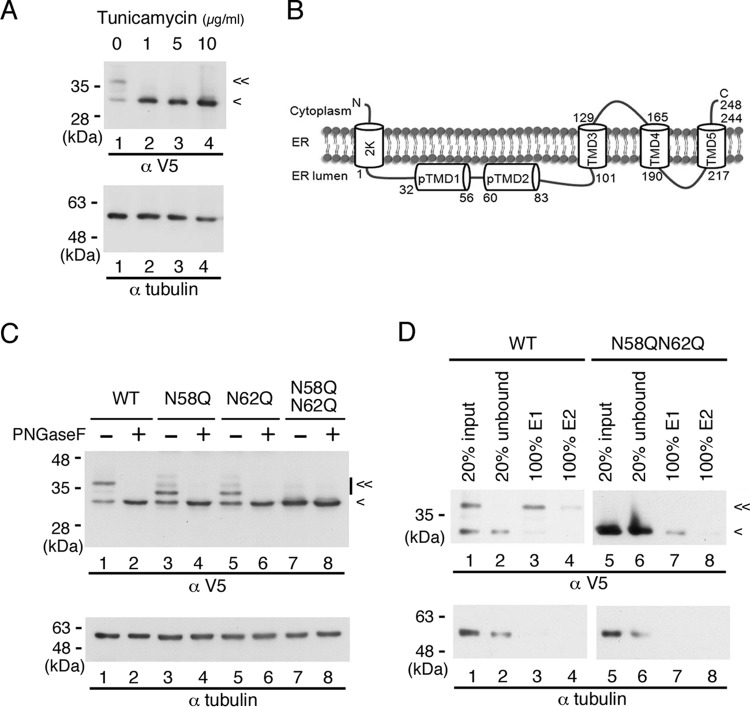
Recombinant NS4B is an N-glycosylated protein. (A) Effect of tunicamycin on recombinant DENV NS4B. HEK-293T cells (2 × 105 cells in a 12-well culture dish) were transfected with p2K-NS4B-V5 (4X) plasmid DNA construct (2 μg) encoding wild-type (WT) sequence. After 6 h posttransfection (hpt), cells were grown in the presence or absence of 0-, 1-, 5-, and 10-μg/ml doses of tunicamycin for 12 h. Cell lysates were harvested, and 10 μg of total protein was resolved by 10% SDS-PAGE. (B) Topology of DENV 2K-NS4B protein. Two domains, pTMD1 and pTMD2, in the ER lumen at the N terminus and three transmembrane domains, TMD3, TMD4, and TMD5, at the C terminus were previously published (14). (C) PNGase F treatment of recombinant NS4B proteins. HEK-293T cells (2 × 105 cells in a 12-well culture dish) were transfected with p2K-NS4B-V5 (4X) plasmid DNA constructs (2 μg) encoding WT, N58Q, N62Q, or N58QN62Q NS4B. Cell lysates were harvested 24 h posttransfection (hpt). Ten micrograms of total protein was treated with or without PNGase F and resolved by 10% SDS-PAGE. (D) Enrichment of recombinant NS4B proteins by ConA agarose. HEK-293T cells (8 × 106 cells in 10-cm culture dish) were transfected with p2K-NS4B-V5 (4X) plasmid DNA construct (10 μg) encoding WT or N58QN62Q NS4B. At 36 hpt, the cells were washed with 1× PBS and lysed in 1.5 ml of 1× PLB. Five hundred micrograms of total cellular protein was applied to ConA agarose for glycoprotein enrichment as described in Materials and Methods. Twenty percent of the starting material (input), 20% of the unbound fraction (unbound), and 100% of the E1 or E2 elution fraction were resolved by 10% SDS-PAGE. In panels A, B, and D, recombinant NS4B protein expression was detected with monoclonal anti-V5 antibody followed by HRP-conjugated anti-mouse antibody. Alpha-tubulin expression served as a loading control. “<” indicates the signal derived from NS4B protein, and “≪” indicates the signal derived from the N-glycosylated form of NS4B protein. All experiments were performed three times independently, and the data shown are from one representative transfection experiment.