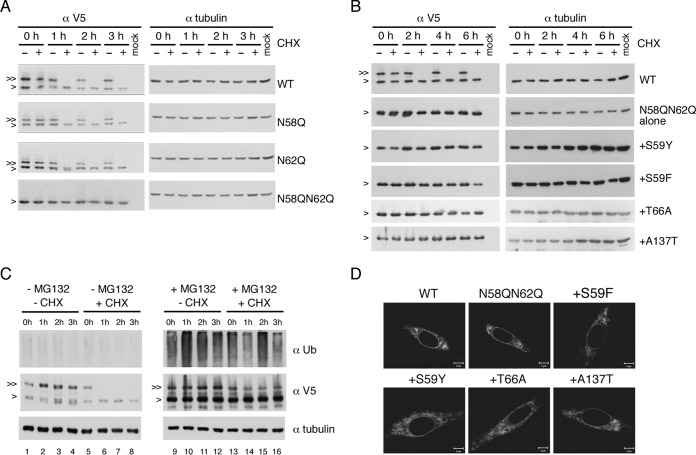
FIG 8.
Ablation of glycosylation did not affect NS4B stability. (A to C) Stability analyses of NS4B proteins. For panels A and B, HEK-293T cells (2 × 105 cells) were transfected with p2K-NS4B-V5 (4X) plasmid DNA constructs (2 μg) encoding WT NS4B or NS4B with N58Q, N62Q, N58QN62Q, or N58QN62Q+suppressor mutations. Cells were treated with 0.5% DMSO or cycloheximide (CHX) (1 mg/ml in 0.5% DMSO) at 24 hpt for 0 to 6 h. For panel C, HEK-293T cells were transfected with p2K-NS4B-V5 (4X) plasmid DNA construct (2 μg) encoding WT NS4B. After 24 h, cells were treated with or without 40 μM MG132 for 12 h and then treated with or without CHX (1 mg/ml) for 0 to 3 h. Cell lysates were harvested, and proteins were resolved by 10% SDS-PAGE. 2K-NS4B-V5 (4X) fusion protein was probed with monoclonal anti-V5 antibody followed by HRP-conjugated anti-mouse antibody. For panel C, the detection of a ubiquitination smear under MG132 treatment indicated the successful inhibition of 26S proteasome machinery and alpha-tubulin expression served as a loading control. “>” indicates the NS4B protein; “≫” indicates the N-glycosylated form of NS4B protein. All experiments were performed twice independently, and the data shown are from one representative transfection experiment. (D) Cellular distribution of NS4B mutant proteins. BHK-21 cells were transfected with p2K-NS4B-V5 (4X) plasmid DNA constructs containing WT, N58QN62Q, or N58QN62Q+suppressor mutations. After 24 h, cells were fixed with 4% paraformaldehyde and the immunofluorescence assay was carried out with anti-V5 monoclonal primary antibody and anti-mouse-Cy3 secondary antibody.