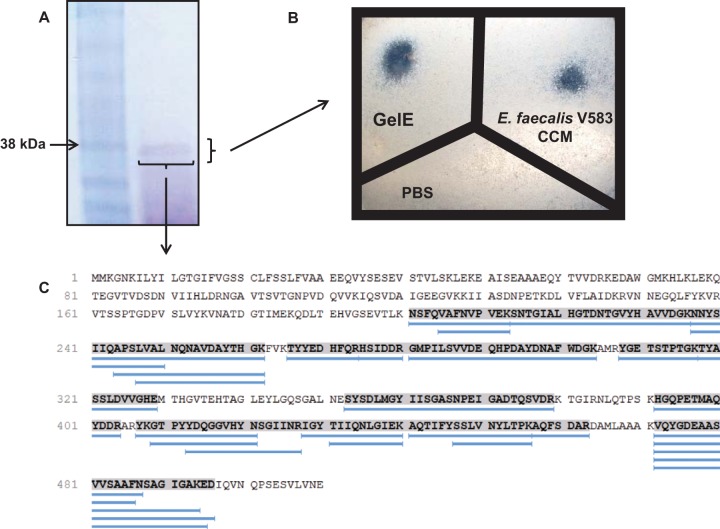
FIG 5.
GelE purified from E. faecalis V583 exhibits caseinolytic activity. (A) Putatively isolated GelE was run on an SDS gel to confirm its size and purity. The isolated protein had a molecular mass of approximately 38 kDa. (B) The putatively purified GelE exhibited caseinolytic activity following overnight incubation on agar plates containing 2.5% skim milk. These data demonstrate that purified GelE retained its functional activity. (C) Alignment of the 27 digested fragments of the putative GelE protein generated by LC–MS-MS. All digested fragments aligned with GelE from E. faecalis V583, confirming the identity of this protein. The shaded sequence indicates the areas homologous to digested fragments. Blue lines represent digested fragments.