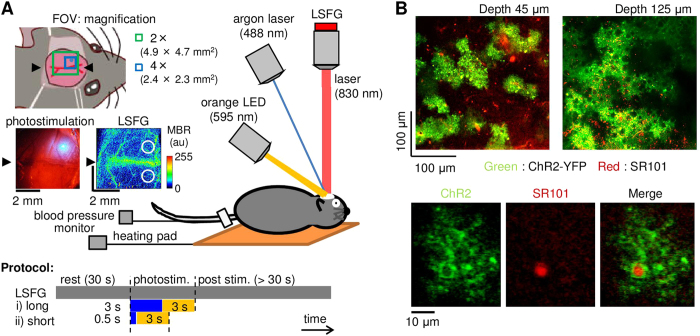
Figure 1.
A) Experimental setup (original drawing). To open ChR2 channels specifically expressed in the astrocytes, an argon laser was induced through an electromagnetic shutter, while spatiotemporal CBF were non-invasively monitored with laser speckle flowgraphy (LSFG), which consisted of an excitation infrared laser and a detection camera (CCD) attached to a microscope. To close the channels, an orange LED was also irradiated following the cessation of the blue laser irradiation. The field of view (FOV) for the LSFG was either 4.9 mm × 4.7 mm or 2.4 mm × 2.3 mm with an objective lens of 2 × (green square) or 4 × (blue square), respectively. A representative spatial arrangement of the irradiated laser spot (0.5 mm in diameter) and a baseline image of the LSFG are shown in bright field and mean blur rate (MBR) images, respectively. A colour bar represents the 8-bit signal level of the MBR. Two circles (1 mm in diameter) in the LSFG image represent the locations of the regions of interest used for calculation of the photostimulation-induced changes in CBF in the ipsilateral and contralateral cortices. Two types of photostimulation were tested: i) long blue laser irradiation (3 sec) followed by a 3-sec orange LED irradiation and ii) short blue laser irradiation (0.5 sec) followed by 3-sec orange LED irradiation. B) Representative images of ChR2-expressing astrocytes (green) co-labelled with sulforhodamine 101 (SR101; red) captured at depths of 45 μm (top left) and 125 μm (top right) from the cortical surface of the extracted brain with two-photon microscopy. The enlarged view (bottom) represents co-localisation (right) of the ChR2-expressing fine processes (left) with the SR101-positive astrocytic soma (middle).