Fig. 10.
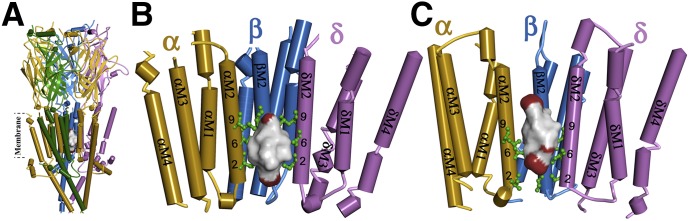
dFBr binding site in the Torpedo muscle-type nAChR ion channel. (A and B) Side view (A) and view from within the ion channel toward the α-β-δ subunits (B) of a nAChR homology model based on the X-ray structure of the mouse serotonin 5-HT3R (PDB ID 4PIR) (Hassaine et al., 2014) and (C) a homology model based on the cryo-electron microscopy structure of Torpedo marmorata nAChR (PDB ID 2BG9) (Unwin, 2005). The nAChR α, β, γ, and δ subunits are colored in gold, blue, green, and violet, respectively. Amino acids photolabeled by [3H]dFBr within the α/β/δM2 helices are colored in green and identified by their position numbers from the N-terminus of the M2 helices. In each model, the ensemble of the 100 lowest energy dFBr docking solutions within the channel is shown as a Connolly surface representation colored by atom (C, black; H, white; Br, red), defining volumes of 905 Å3 (B) and 821 Å3 (C) compared with the dFBr molecular volume of 241 Å3.
